Background
The HEp-2 indirect immunofluorescence assay (IFA) is the reference method for autoantibody screening, guiding antigen-specific testing and titer assessment. ANA titers ≥1:320 are strongly associated with systemic connective tissue diseases (1,2). Harmonization initiatives include the Brazilian Consensus (2000) (3) and the International Consensus on ANA Patterns (2014) (4). The third BCA (2009) recommended conjugate titer calibration and use of reference sera in quality assessments (5). This study assesses the reproducibility of HEp-2 titer determination in 67 Brazilian laboratories.
Aim
To evaluate the reproducibility of HEp-2 autoantibody titer determination among Brazilian clinical laboratories participating in an external quality assessment program.
Methods
Fifteen serum samples, covering four defined reactivity ranges (negative, low, moderate, and high), were sent to 67 Brazilian clinical laboratories for HEp-2 autoantibody titer characterization. Results within one dilution above or one dilution below the nominal titer were considered acceptable. Concordance, discordance, overestimation, and underestimation indices were calculated based on the proportion of laboratories within or outside the acceptable range, with values representing the average of three evaluations per range. Sample distribution was coordinated by Controllab, a Brazilian EQA provider.
Results
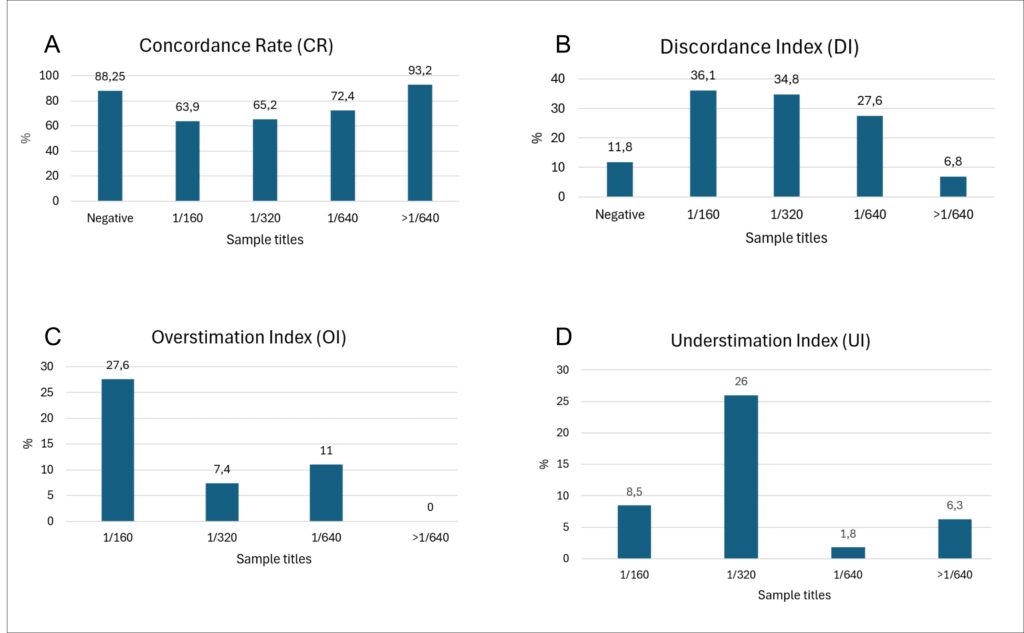
The mean concordance rate (CR) for negative patterns was 88.25%, with 11.8% of laboratories incorrectly assigning titers ≥1/80 (Discordance Index, DI). Accuracy was lowest for titers of 1/160 and 1/320, with CR values of 63.9% and 64.1%, respectively, and highest for 1/640 (72.4%) and >1/640 (93.2%). DI was lowest for negative samples (11.8%) and >1/640 (6.8%), but reached 27.6 – 36.1% for 1/160–1/640. Overestimation was most frequent for 1/160, whereas underestimation predominated for 1/320.
Conclusions
The study concluded that, while the identification of negative samples demonstrated high concordance (88.25%), inter-laboratory reproducibility varied significantly according to antibody titer. Low-titer samples (1:160 and 1:320) exhibited reduced concordance and higher discordance rates compared to high-titer samples (1:640 and >1:640), rendering them more susceptible to misclassification. Such discrepancies, particularly within the intermediate titer range, underscore the necessity for enhanced standardization to ensure greater consistency and reliability of results across laboratories.
Table 1: Results of the average of three assessments of samples at different concentration ranges, expressed as low, intermediate,
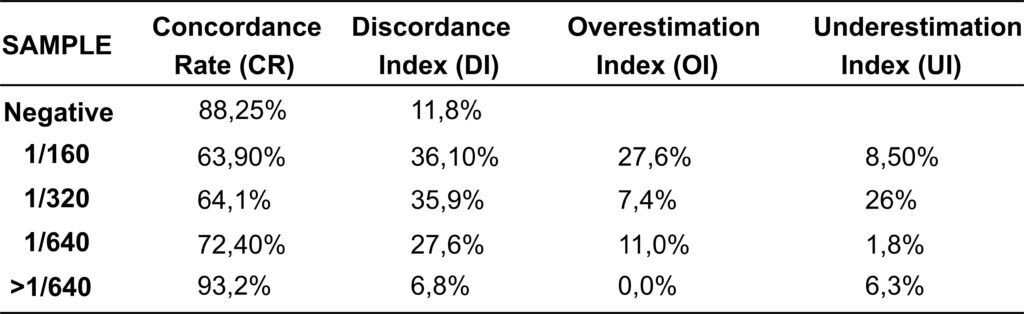
Figure 1. Average of three assessments of samples across different concentration ranges (negative, low, intermediate, and high titers), showing the concordance rate, discordance index, overestimation index, and underestimation index for titer classification among 67 Brazilian clinical laboratories.
Disclosure
The authors confirm that they don’t have any conflict of interest to declare.
References
1. Almagro R, Rodríguez Gutiérrez JF, Marơn-Marơnez MA, Rodríguez Valls MJ, Aranda Valera C, de la Iglesia Salgado JL. Association between antinuclear antibody titers and connective tissue diseases in a Rheumatology Department. Reumatol Clin. 2017 May-Jun;13(3):150–5. doi:10.1016/j.reuma.2016.03.019. PMID: 27221374.
2. Agustinelli RA, Rodrigues SH, Mariz HA, Prado MS, Andrade LEC. Distinctive features of positive anti-cell antibody tests (indirect immunofluorescence on HEp-2 cells) in patients with non-autoimmune diseases. Lupus. 2019 Apr;28(5):629–34. doi:10.1177/0961203319838348. Epub 2019 Apr 26. PMID: 31027463.
3. Dellavance A, Gabriel Júnior A, Cintra AFU, Ximenes AC, Nuccitelli B, Taliberti BH, et al. II Consenso Brasileiro de Fator Antinuclear em Células HEp-2: Definitions for standardization of autoantibody testing against the nucleus (ANA HEp-2), nucleolus, cytoplasm and mitotic apparatus, as well as its clinical associations. Rev Bras Reumatol. 2003 May;43(3):129–40. Available from: hƩps://www.scielo.br/j/rbr/a/97swXNNt6zqDGVSxcyvXJ4D/
4. Chan EKL, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PLC, et al. Report of the first internaƟonal consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014–2015. Front Immunol. 2015;6:412. doi:10.3389/fimmu.2015.00412.
5. Dellavance A, Gabriel Júnior A, Nuccitelli B, Taliberti BH, von Mühlen CA, Bichara CDA, et al. 3º Consenso Brasileiro para pesquisa de autoanticorpos em células HEp-2 (FAN): recomendações para padronização do ensaio de pesquisa de autoanticorpos em células HEp-2, controle de qualidade e associações clínicas. Rev Bras Reumatol. 2009 Mar;49(2):89–98. doi:10.1590/S0482-50042009000200002.