Background
The glycated hemoglobin (HbA1c) is an essential parameter for managing diabetes mellitus. The National Glycohemoglobin Standardization Program (NGSP) and the International Federation of Clinical Chemistry (IFCC) were essential for the harmonization process to reduce the variability of measurements. In Brazil, Controllab, accredited by ABNT NBR ISO/IEC 17043:2011, in partnership with the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML), provides an External Quality Assessment Program for HbA1c.
Aim
The objective of this study was to evaluate the performance evolution of laboratories participating in this External Quality Assessment Program from 2009 to 2022.
Methods
The performance of methods harmonized and not-harmonized by NGSP, IFCC, and IFCC/NGSP were evaluated. The methods were: boronate affinity chromatography (BAC), ion exchange chromatography (IEC), electrophoresis (EP), direct (DR), enzymatic (EZ), photometry (PT), ion exchange HPLC (HPLC), immunofluorescence (IF), immunoturbidimetry (IT), turbidimetry (TB) and chemiluminescence (CL). Standard descriptive analyses were carried out. The Kruskal-Wallis test was used for di erences between two or more groups of an independent continuous variable. The Mann-Kendall statistical test was used to assess whether there was a trend in a set of data values and whether the trend was statistically significant. The RStudio software was used for the data analysis.
Results
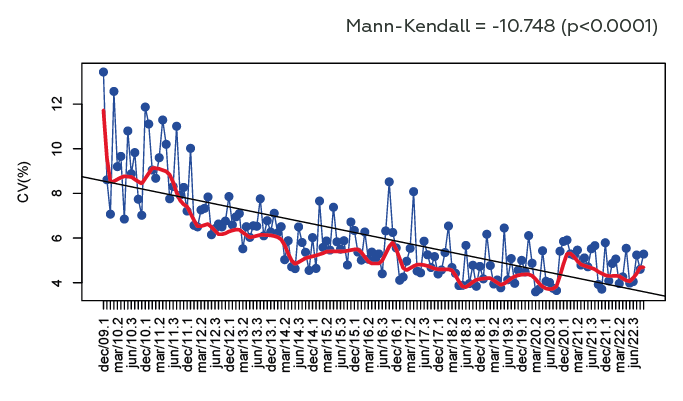
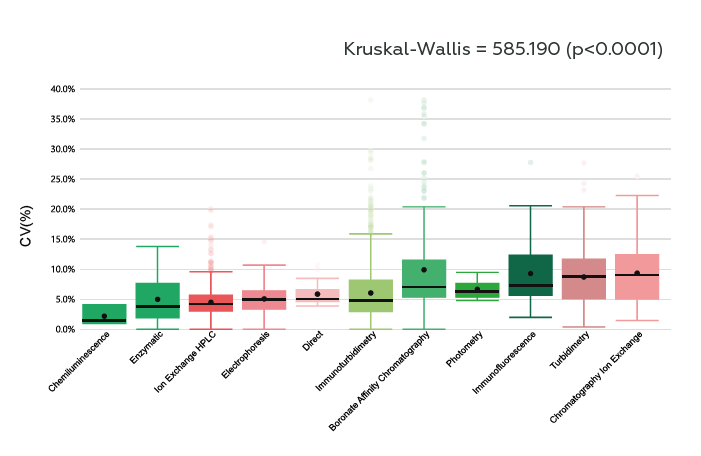
A total of 771 laboratories (706 from Brazil and 65 from other countries) submitted 43960 datasets. In 2009, the coefficient of variation (CV) among laboratories was 8.6%, and in 2022 the CV was 4% (p<0.0001) (Figure 1). The methods that presented the lower median CV were CL (1.5%), EZ (3.8%) and HPLC (4.2%), and the higher median CV was observed in IEC (9.1%), TB (8.8%) and IF (7.35%) (p<0.0001) (Figure 2).
In 2010, the proportions of laboratories using methods harmonized by NGSP, IFCC and IFCC/NGSP were 21%, 22.22%, and 30.45% respectively; for the laboratories using methods not-harmonized was 26.34%. In 2022, these proportions were 18.32%, 21.20%, and 42.41% respectively, and for laboratories using methods not-harmonized was 18.06%. The reduction in the proportion of methods not-harmonized and the increase in the proportion of laboratories using methods harmonized by both IFCC/NGSP was statistically significant (P<0.05). The median of CV of methods not-harmonized was 8.15% and for methods harmonized was 4.8% (IFCC), 5.2% (NGSP), and 4.7% (IFCC/NGSP) (P<0.0001).
Conclusion
There was a reduction in the median CV from 2009 to 2022 and in the proportion of laboratories using methods not-harmonized during the same period. Also, the median CV of methods not-harmonized was higher than methods harmonized. These findings could raise the hypothesis that harmonization of HbA1c improves lab performance and could impact the quality of routine clinical results.
Disclosure
The authors confirm that they don’t have any conflict of interest to declare.
Bibliographic References
Oliveira CA, Mendes ME (orgs.). Gestão da fase analítica do laboratório: como assegurar a qualidade na prática. 1 ed., v. 2. Rio de Janeiro: Controllab: 2011, 184p.
ISO/IEC 17043:2010 – Conformity assessment – General requirements for proficiency testing.
Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013 Nov; 33(6):393-400. doi: 10.3343/alm.2013.33.6.393. Epub 2013 Oct 17. PMID: 24205486; PMCID: PMC3819436.
English E, Lenters-Westra E. HbA1c method performance: The great success story of global standardization. Crit Rev Clin Lab Sci. 2018 Sep; 55(6):408-419. doi: 10.1080/10408363.2018.1480591. Epub 2018 Jul 12. PMID: 30001673.
The role of the Brazilian proficiency testing/External Quality Assessment Program in the improvement of glycated hemoglobin measurement
- Claudio Bastos, Nairo M. Sumita, Adriana O. Vieira, Maria Elizabete Mendes, Rafael M. Lopes, Rafael N. Moresco, Katia Nery, Bruno C.A. Souto-Santos, Fábio V. Brazão and José Antonio T. Poloni