Controllab’s pioneering initiative ensures consistent performance of rapid tests and enhances diagnostic safety.
During the 57th Brazilian Congress of Clinical Pathology/Laboratory Medicine (57th CBPC/ML), held from September 16 to 19 in Rio de Janeiro, the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML) and the Brazilian Chamber of Laboratory Diagnostics (CBDL) formally expressed institutional support for the Batch-to-Batch Certification Program — a pioneering Controllab initiative aimed at strengthening the quality of rapid tests.
This action underscores the relevance of the initiative and the organizations’ commitment to technological and regulatory advancement, while also driving technical and scientific improvement in the field of in vitro diagnostics for the benefit of public health.
Consistency assurance in every batch
Even with strict production controls, variations between reagent batches can occur and may affect analytical performance — and, consequently, confidence in the results.
The Batch-to-Batch Certification Program takes a preventive approach, providing greater safety for laboratories and healthcare services performing the analyses. Before a batch is used in routine testing, the certification issued by the manufacturer confirms that it has undergone an independent evaluation, ensuring consistent and reliable results.
The certificate is made available free of charge by the manufacturer to the facilities performing the tests, reinforcing a commitment to transparency and quality.
This practice ensures:
- Greater confidence for professionals.
- Greater safety for patients.
- Greater efficiency throughout the entire care journey.
An initiative that inspires trust
According to Alvaro Pulchinelli Junior, President of SBPC/ML, “The support for the Batch-to-Batch Certification Program reflects SBPC/ML’s commitment to excellence and safety in laboratory diagnostics. The initiative aligns with the best practices recommended by the Society, strengthening traceability and the reliability of results — both essential for patient safety and quality of care.”
For Fúlvio Facco, Chairman of the CBDL Board, this type of partnership enables test manufacturers to comply with relevant health regulations, which has greatly contributed to increased product availability for the population.
“CBDL and its members are pleased with this initiative because it allows for greater public access to Point-of-Care testing. Both manufacturers and healthcare providers will be working in compliance with ANVISA’s new regulation.”
According to Controllab’s CEO, Vinicius Biasoli, the certification represents more than a technical validation — it marks an evolution in the culture of quality: “Our commitment has always been to strengthen confidence in laboratory results. With Batch-to-Batch Certification, we deliver quality and safety to test users, supporting more accurate clinical decisions and more efficient routines. Innovation, quality, and regulatory compliance can move forward together, raising the standard of excellence across the entire sector.”
Certification that benefits everyone
Benefits for organizations performing tests
Include batch certification in your routine and achieve:
- Safety when changing batches
- Fewer internal checks
- Reduced costs and quality checks
- More consistent and reliable results
- Compliance with standards and regulations
Healthcare services,
how to obtain the certificate?
Simply request it from the manufacturer — the certificate is free for you.
Benefits for manufacturers and registration holders
Certify your product batch and strengthen your brand’s reputation:
- Technical validation of batch performance
- Transparency with customers
- Competitive advantage
- Reduction of post-sale issues
- Meets the needs of testing laboratories, reducing their costs and efforts when changing batches
Manufacturer,
how to obtain the certificate?
Contact Controllab.
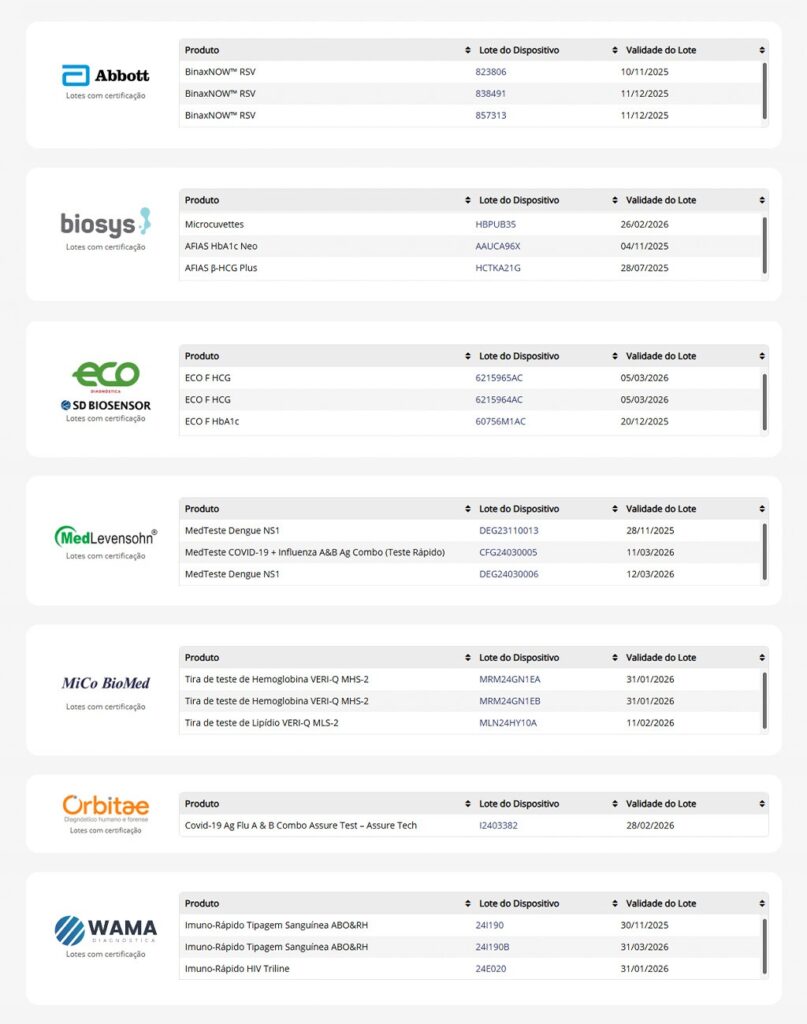
Manufacturers that have already joined
Want to know which manufacturers are already participating in the program?
Check the table below:
A trusted network that transforms care
The statements from SBPC/ML, CBDL, and Controllab converge on one point: ensuring reliable results is a shared commitment.
Batch-to-Batch Certification not only raises the technical standard of POCTs but also strengthens trust across the entire diagnostic chain — from the manufacturer to the healthcare professional, and most importantly, to the patient.
Adopt this quality practice
Want to have this advantage in your routine? Speak with the manufacturer of your rapid tests and request batch certification.
This choice provides greater confidence in the results and reinforces your service’s commitment to reliable diagnostics.

