Controllab recognition highlights high standards of analytical quality.
The reliability of results is built every day. It depends on well-defined processes, continuous monitoring, and a permanent commitment to analytical quality.
In this context, Controllab clients have already received the 2025 Proficiency Certificate, a recognition granted to those who participated in Proficiency Testing (PT) programs throughout the period and achieved the expected analytical performance. More than a technical document, the certification conveys to the market the seriousness with which these organizations conduct their analytical routines.
Independent assessment that underpins quality
Proficiency Testing — also known as External Quality Assessment (EQA) — provides an independent reference for monitoring analytical performance. Through scheduled rounds, organizations analyze control samples and have their results evaluated based on previously defined technical criteria.
Comparison with other participants and careful data analysis make it possible to identify deviations, correct failures, support technical decisions, and strengthen compliance with regulatory and normative requirements. This process directly contributes to continuous improvement and to the safety of the services provided.
A certification recognized by the sector
The 2025 Proficiency Certificate covers national and international organizations in the clinical, veterinary, microbiology, hemotherapy, and physicochemical segments.

In the clinical segment, it is endorsed by the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML). In the veterinary segment, the certification is endorsed by the Brazilian Society of Veterinary Medicine (SBMV), reinforcing its technical relevance and alignment with best practices in each field.
This technical recognition positions analytical services in a differentiated way before clients, partners, and other key stakeholders.
When certification goes beyond the technical environment
Although it originates from a rigorous analytical assessment, the value of the Proficiency Certificate goes beyond the analyses themselves. For the market, it represents transparency, care, and confidence in the results delivered.
Laboratories and other analytical services that clearly communicate their continuous participation in external quality assessments strengthen their institutional reputation and enhance the perception of credibility among their clients.
Your quality is already recognized. Now it’s time to show it.
To support clients in this process, Controllab provides guidance content with best practices, examples, and guidelines on how to communicate the meaning of the Proficiency Certificate in a clear and strategic way.
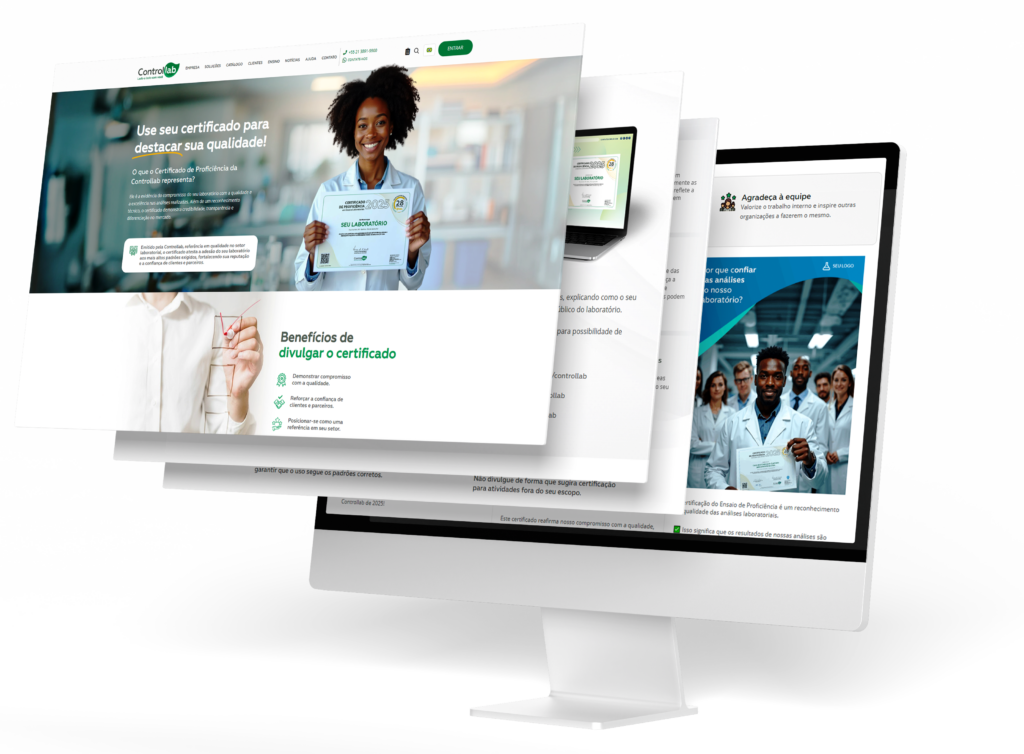
The material helps translate technical criteria into accessible messages, bringing the organization closer to its audience and highlighting its achievements.
For laboratories and other analytical services that do not yet participate in Controllab’s Proficiency Testing programs, the certification represents a concrete opportunity to strengthen processes, demonstrate commitment to quality, and gain greater market confidence.
Learn more about quality control programs at controllab.com.

