OPTIMIZE THE FEATURES OF YOUR ROUTINE!
Controllab maintains the microorganisms for its laboratory.
To optimize time in the laboratory routine, Controllab ships certified strains that replace the passages. They are strains for prompt delivery, in the amount defined by the laboratory, from the selected microorganisms.
Why its important?
Certified strains confer more confidence and credibility in quality control, facilitating the process to comply with Anvisa / VISA standards, audits, accreditations and inspections.
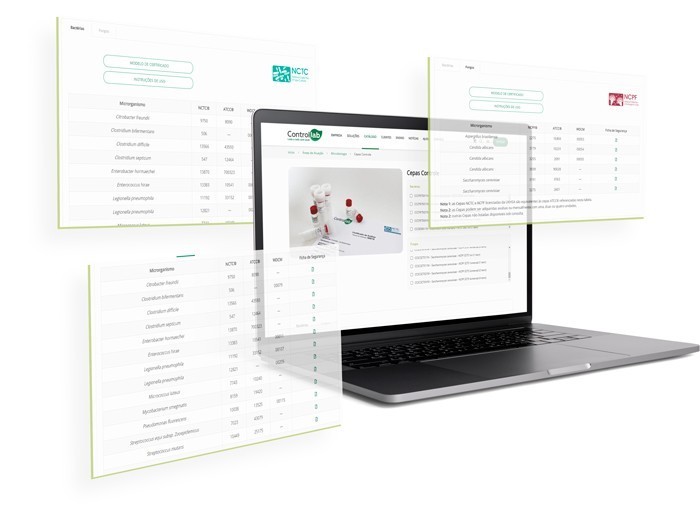
NCTC® vs. ATCC®
Understand the equivalence of the main culture collections
NCTC®, like ATCC®, is a global reference for culture collections. Both are registered in the WDCM (World Data Center for Microorganisms), an international database that groups strains into a unique identifier system, facilitating the comparison and sharing of information on reference strains.
In the WDCM, the same strain can have different designations depending on the collection. For example, the Escherichia coli strain NCTC® 12241 is also designated as ATCC® 25922; CIP® 76.24; DSM® 1103; CCUG® 17620; CECT® 434; depending on the culture collection.
Therefore, when using an NCTC® or ATCC® strain, you are using reference strains with the same phenotypic characteristics and genotyping. It is worth noting that technical protocols and accrediting bodies recognize all available culture collections, ensuring equivalence and compliance among them.

There are seven steps in the routine of raise production: reconstitution of the inoculum, sowing, incubation, cryoprotection, sterilization of flasks, freeze-drying and freezing for conservation. With the REFERENCE STRAINS Program, your laboratory will be responsible only for the reconstitution of the inoculum, sowing and incubation. The rest are up to Controllab.
ADVANTAGES OF ACQUIRING THE PROGRAM:
- Immediate delivery
- More agile processes
- More time to devote to analysing the results
- Maximization of infrastructure resources
- Less time with records for audits
- Cost optimization
- Ending of passage preparation
Time
Costs
Infrasctruture
Documents
Laboratory benefits
Confidence in microbiological analysis
Most of the strains supplied are first generation, or at most up to the third generation, avoiding mutations and phenotypic changes that can affect the results obtained in the analyzes.
This definition of generations complies with the various standards for accreditation processes of laboratories and regulatory bodies in the area.
Credibility in Quality Control
The laboratory enrolled in the program uses reference strains certified with quality and international recognition.
The lyophilized bacteria strains originate from the oldest culture collection in this category NCTC (National Collection of Type Cultures).
Scheduled supply of lyophilized strains
By joining the program, there is a continuous supply of the number of strains the laboratory needs, according to the schedule determined by the client.
The laboratory also has immediate access to extraordinary purchases, due to the large and secure strain deposit of Controllab.
Productivity increase
With the maintenance of microorganisms and the scheduled supply of strains, the teams involved in the analysis and acquisition processes gain more time to dedicate themselves to other routine activities.
Check the full list of available strains
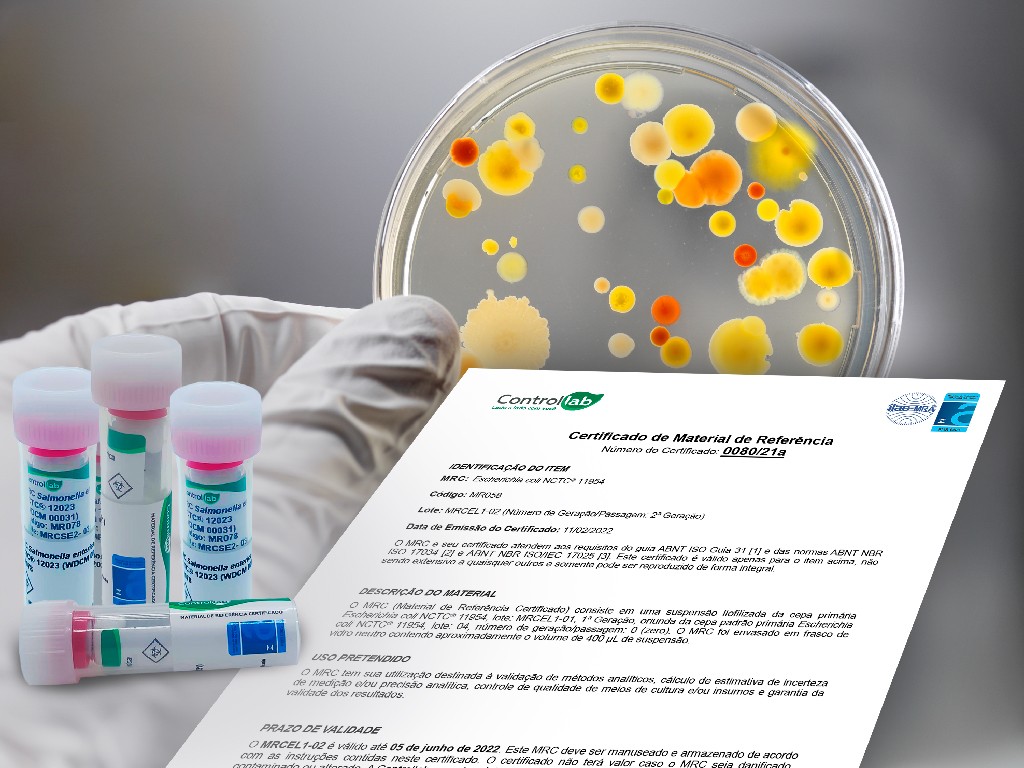
In addition to the benefits and advantages provided by the Program, Controllab provides strains certified by the NBR ISO 17034 standard, which are recognized as Certified Reference Material (MRC), the main quality standard within a laboratory’s analytical routine.
Controllab and UKHSA
Controllab, in its continuous search to provide the best solutions for laboratory quality control and attentive to laboratory challenges to remain sustainable and optimized resources, is expanding the initiatives in the microbiological area and begins these actions with the collaboration of the UKHSA – UK Health Security Agency.
A UKHSA is the custodian of four unique collections, consisting of authentically preserved cell lines and microbial strains of known origin.
Among the collections, the NCTC (National Collection of Type Cultures) is the oldest of its kind – founded in 1920 – and has strains registered at the World Data Center for Microorganisms (WDCM).

Authenticated reference strains and internationally recognized quality standards
The Reference Strains Program is born with the commitment to deliver strains most of the 1st generation or up to the 3rd generation to laboratories. This commitment will help organizations to efficiently comply with the various regulations in the microbiological area for laboratory accreditation processes and regulatory agencies.
Authenticated reference strains are of paramount importance for the control of clinical diagnostic exams. By enrolling in the Strains Control Program, the laboratory tem acesso a um service with internationally recognized quality standards and immediate delivery.
Controllab has been accredited since 2016 as a Certified Reference Material Producer by the Coordenação-Geral de Acreditação do Inmetro, under number PMR 0009, according to the scope published at www.inmetro.gov.br.
Why should I join the Reference Strains Program?
Efficiency
Controls exam quality processes with authenticated strains
Sustainability
Perpetuates lab quality and credibility
Quality
Contributes to the efficiency of analysis processes
Traceability
Accompanies certificates that prove the authenticity of the microorganism
Authenticity
Uses strains from internationally recognized culture collection
Reliability
Promotes confidence in the microbiological processes
The laboratory enrolled in the REFERENCE STRAINS Program stops performing routine maintenance (continuous passages). Controllab provides this maintenance along with the selected strain certificate.
This practicality allows the laboratory to simplify the routine, gain more agility and reduce costs and time involved in testing and recording to ensure the quality of the peals in the process and to be audited.
See also the Gram and BAAR controls
This sustainability and quality is also available through Gram and BAAR controls
How does it help in my laboratory?
The strains of the Gram and BAAR controls are applied to the slides for positive and negative control of related microorganisms, which help laboratories to evaluate new batches of dyes / reagents.
The controls consist of 15 slides of:

Gram CONTROL
Smeared slides containing Staphylococcus aureus and Escherichia coli microorganisms.

BAAR CONTROL
Smeared slides containing Mycobacterium tuberculosis and Escherichia coli microorganisms.
Pioneering spirit, trust, and innovation that transform analytical results.
Access our corporate brochure and see how we make a difference.