During the largest congress in the sector, the company unveiled initiatives that enhance the safety and efficiency of analytical routines.
Controllab, the leading provider of quality control solutions in Latin America, reinforced its pioneering position during the 57th Brazilian Congress of Clinical Pathology/Laboratory Medicine (57º CBPC/ML), held from September 16 to 19 at Riocentro (RJ).
The new developments drew the attention of participants for their potential to transform analytical workflows, providing greater confidence for managers and teams. Some of the innovations are already available to clients in the clinical segment, boosting efficiency and process safety.

Multi-equipment reports: before and after interlaboratory comparison
Reporting results from multiple systems has long been part of the routine for Controllab’s Proficiency Testing users, even before the Brazilian standard RDC 978/2025. Now, this practice has been enhanced with the addition of a real-time intralaboratory report.
By entering results from different systems for the same test, participants can immediately view a comparison between their instruments, identifying any discrepancies while the external control is still being processed. After the round is closed, the data is compared with market results, offering a broader and more strategic view.
As with multi-system reporting, access to the new report is free for all participants.
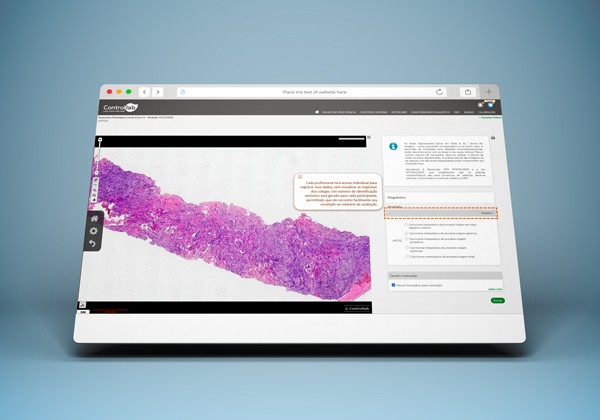
Multi-microscopist reporting: better-aligned teams
Also available at no additional cost, this feature allows multiple microscopists to submit results for the same case in the Proficiency Testing program.
The quality control administrator receives a consolidated view of the data, making it easier to identify improvement opportunities and strengthen team performance.
This functionality is a valuable support tool for laboratories seeking continuous professional development.
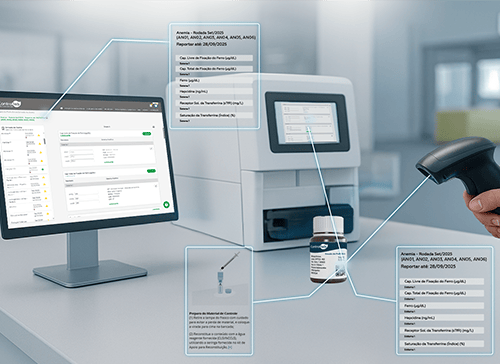
Proficiency Testing automation for greater day-to-day agility
One of the event’s biggest highlights was the automation of the Proficiency Testing reporting process, developed through a partnership between Data Innovations and Controllab.
This world-first initiative connects the sample, laboratory software, and Online System, ensuring agility and security in result submission. Already being implemented at an Einstein Hospital Israelite unit, the solution was described by Alice Friedenberg, Sales Executive at Data Innovations for Latin America, as: “An example of the power of collaboration to turn data into decisions and elevate laboratory quality standards.”
At the booth, visitors also had a first look at another innovation: QR Code labels on materials, giving instant access to control instructions and test lists for a more practical workflow.

Analytical indicators in Metricare: intelligence for strategic decisions
Metricare, Controllab’s data intelligence platform, now features an Analytical BI module dedicated to quality control. The feature automatically structures Proficiency Testing data, enabling strategic comparisons across single or multiple lab networks.
The tool is already being used by Rede D’Or São Luiz. During the congress, Dr. Nilo Duarte, Head of Clinical Analysis in São Paulo, shared his experience in a workshop: “Controllab solved a problem for me. With Analytical QC BI, we can view performance from a broader perspective and act quickly, strengthening quality management.”

Batch-to-Batch Certification for POCTs: confidence before use
One of the highlights for the In Vitro Diagnostics audience was the institutional support from SBPC/ML and CBDL for the Batch-to-Batch Certification Program. Their endorsement reinforces the relevance of the initiative and the sector’s commitment to exam safety and reliability.
Each new batch is evaluated in advance at no cost to healthcare facilities. Certification is provided directly by the manufacturer, ensuring consistent performance across batches and preventing undesirable variations.
This practice delivers clear benefits: greater uniformity, cost savings, and compliance with regulations such as the Brazilian standard RDC 978/2025. The result is confidence for professionals and protection for patients throughout the care journey.
To access the certificate, simply request it from the manufacturer — it is free for testing services.
A trusted partner in quality control
The solutions presented reaffirm Controllab’s commitment to anticipating trends and supporting safe clinical decision-making, always with a focus on excellence in results. More than innovations, attendees witnessed practical examples of how the company transforms industry challenges into accessible and reliable solutions.
Already a Controllab client?
Take advantage of these updates to bring even more efficiency and confidence to your routine.
Not yet partnered with Controllab?
Talk to a specialist and discover how to strengthen the quality and recognition of your service.

