Greater speed and confidence in decision-making with third-party control, interlaboratory comparison, and results monitoring.
Laboratory diagnostic facilities undertake a continuous commitment to ensure the reliability of their results. To maintain this trust and consistency in analytical processes, participation in an Internal Quality Control (IQC) program is essential.
With 48 years of experience and presence in over 40 countries, Controllab offers the Internal Control (IC) program — a tool that promotes continuous monitoring of reproducibility during the analytical phase in the laboratory. The program helps identify and eliminate errors inherent to both quantitative and qualitative assays.
Why choose Controllab’s Internal Control?
- Third-party (independent) controls.
- Samples evaluated through interlaboratory comparison.
- Free access to CI ONLINE with application of Westgard rules, Levey-Jennings charts, and performance history on results.
Confidence and accuracy with third-party controls and interlaboratory evaluation
Third-party internal controls are prepared by suppliers different from the manufacturers of the routine reagents or calibrators. These materials, also known as independent or unbiased controls, ensure a reliable assessment of analytical performance.
According to ISO 15189, laboratories are recommended to consider using independent, third-party IQC materials — either as a replacement or in addition to the controls provided by the reagent or instrument manufacturers themselves. These controls serve to:
- Independently verify analytical performance.
- Detect failures not visible with manufacturer controls.
Meet regulatory requirements and strengthen accreditation processes.
All control materials offered by Controllab are third-party, contributing to safer, more reliable laboratory practices that align with international standards.
They contain clinically relevant concentrations that simulate real conditions in the laboratory routine. Most of these materials come from Proficiency Testing (PT), which ensures interlaboratory evaluation validated across different analytical systems.
Expanded portfolio for POCT routines
In addition to supporting automated routines and other methodologies, Controllab also offers a broad range of internal controls for POCT (Point-of-Care Testing / Rapid Tests).
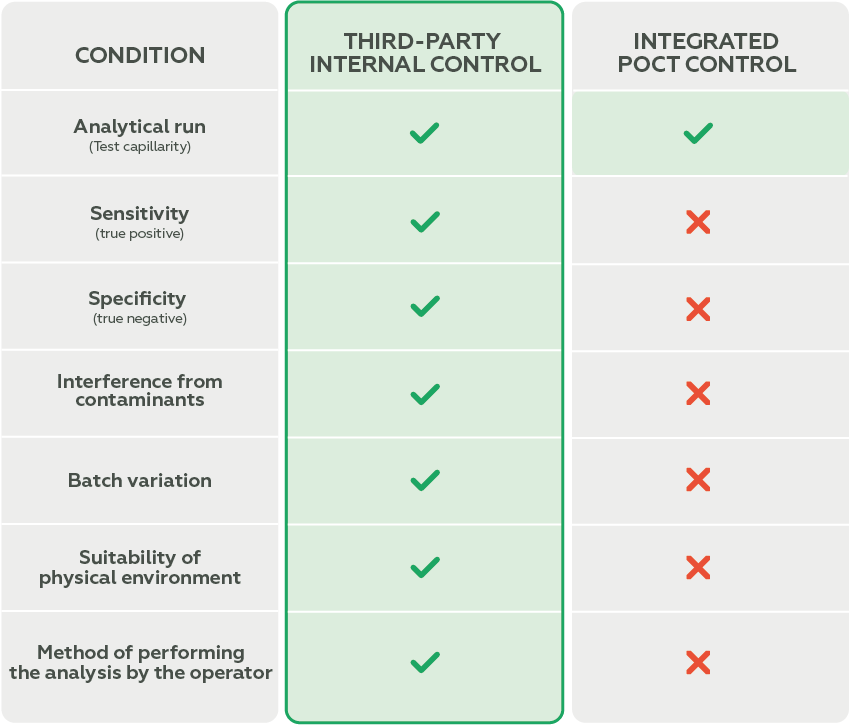
Although these devices typically come with a built-in verification system (integrated control), it is essential to understand the difference between Internal Quality Control and Integrated Control.
Integrated control performs automatic checks within the analytical system itself and is common in POCT equipment. It monitors, for example:
- Proper sample flow;
- Reagent functionality;
- System integrity.
However, integrated control does not evaluate critical parameters such as:
- Sensitivity (ability to detect true positives);
- Specificity (ability to detect true negatives).
Internal Quality Control, on the other hand, is performed with external materials that simulate real patient samples. It allows for an independent and more comprehensive assessment of the analytical system’s performance, including:
- Sensitivity and specificity of reagents;
- Temperature variations;
- Handling errors;
- Reagent degradation.
Does integrated control replace internal control?
No. While integrated controls are useful, they do not replace internal control. They should be used as complementary tools.
To ensure result reliability, the ideal approach is to:
- Use the integrated control to check system functionality.
- Apply internal control to validate actual performance and identify deviations the system may not detect.
Want to enhance safety and accuracy in your analytical routine?
To achieve that, it’s important to remember that Internal Quality Control (IQC) does not replace the Proficiency Testing (PT), also known as External Quality Assessment (EQA). These tools complement each other, with PT being essential for evaluating the effectiveness of continuous monitoring.
Talk to a Controllab specialist now or click here to learn more about the Internal Control Program.

