The programs provided by the company are essential to ensure the accuracy, reliability, and consistency of the results of this diagnostic modality.
Technological advancements in laboratory medicine have played a crucial role in the miniaturization of diagnostic equipment. This modernization is revolutionizing the way primary healthcare is delivered.
In this context, Point-of-Care Testing (POCT) refers to diagnostic tests performed directly at the patient’s location. These POCTs provide rapid results that assist healthcare professionals.
What is the importance of quality control for POCT?
POCTs fall under the category of in vitro diagnostic (IVD) products and include reagents, substrates, buffer solutions, and other consumables necessary for the analysis.
Although they are designed to be accurate, it is essential to use quality control to monitor the analytical performance of these devices, thereby ensuring the reliability and effectiveness of the tests.
Quality control is carried out using Internal Quality Control (IQC) and Proficiency Testing, also known as External Quality Control (EQA):
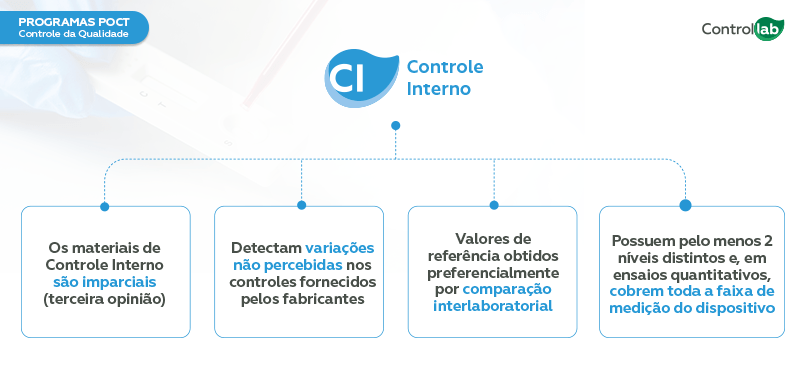
Internal Quality Control (IQC) – Monitors the analytical routine of testing facilities, preventing errors and quality deviations from impacting patient results.
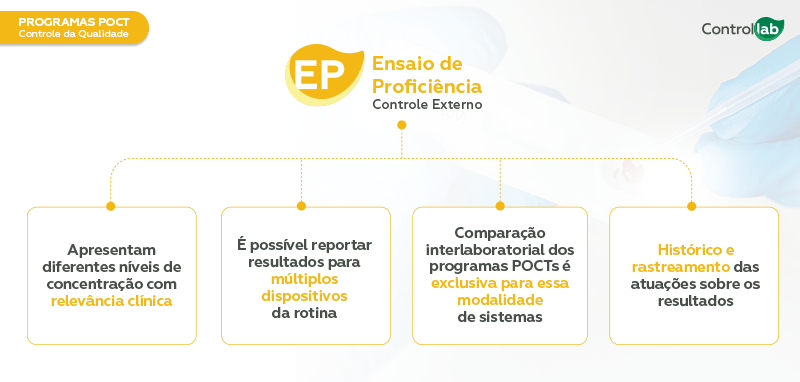
External Quality Control (EQA) – Periodic interlaboratory comparison of results to assess the accuracy of the results and the effectiveness of monitoring analytical processes.
Do POCTs with integrated control also need to be monitored by quality control?
It is common for POCTs to have integrated (built-in) control that performs an electronic check of the device and sample flow, indicating whether all present reagents are active and functioning correctly. However, integrated control does not monitor all conditions that may affect test results.
The use of impartial internal controls (third-party samples), which simulate patient samples, is essential for monitoring the device’s performance and identifying potential issues that could compromise patient safety in advance.
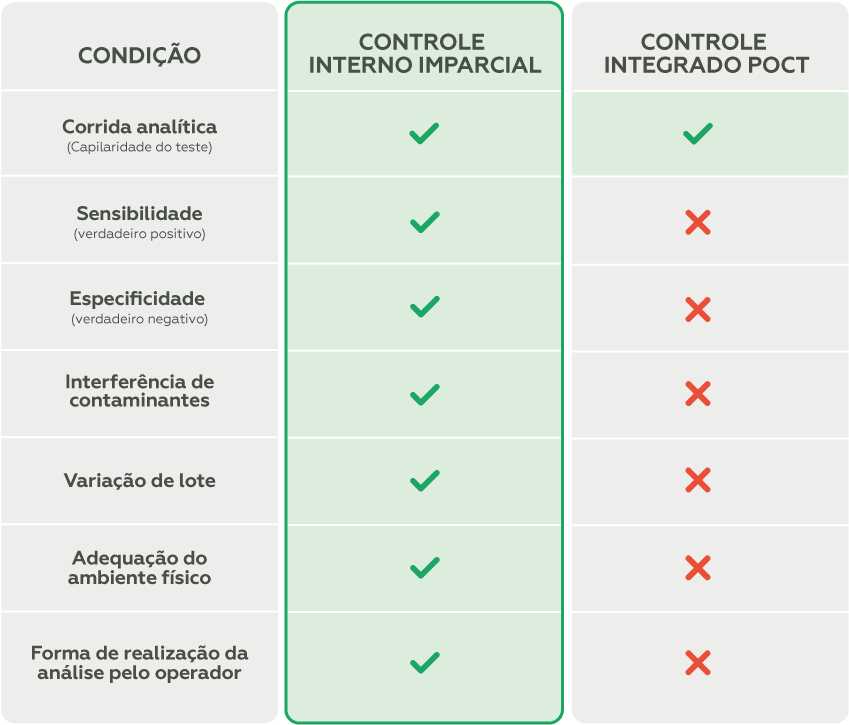
To maximize the identification of the conditions described in the table above, it is essential that impartial internal controls are used in conjunction with external quality control.
What controls are provided by Controllab?
Controllab offers external controls (Proficiency Testing) and internal controls that elevate the standards of POCT analytical routines. It is worth noting that the programs are specific to POCTs. The samples are stable, easy to use, and reflect the clinical conditions of patients. Below are the specifications related to each of these controls:
Expansion of quality control programs for POCTs
Controllab’s external controls are conducted in accordance with ISO 17043. Both these and the internal controls provided by the company are designed to meet national regulatory requirements, such as RDC 786/2023, as well as international standards. This ensures confidence in the use of these devices.
To promote the improvement of the quality of healthcare services provided to the public and the analytical reliability of facilities, Controllab offers new controls, including: POCT Hematology; POCT Cystatin; and POCT Qualitative Procalcitonin, detailed as follows:
| Programas | Ensaios disponíveis |
|---|---|
| POCT Hematologia | Série Vermelha, Parâmetros Plaquetários e Série Branca |
| POCT Cistatina C | Cistatina C |
| POCT Procalcitonina Qualitativo | Procalcitonina Qualitativo |
In addition to these programs, Controllab offers a wide range of controls for POCT, promoting reliable monitoring of routines related to rapid tests.

Do you want recognition for excellence in your Point-of-Care reports?
Speak now with a Controllab specialist, boost your facility’s reputation, and enhance your audience’s satisfaction.