EP
CI
CLÍNICO
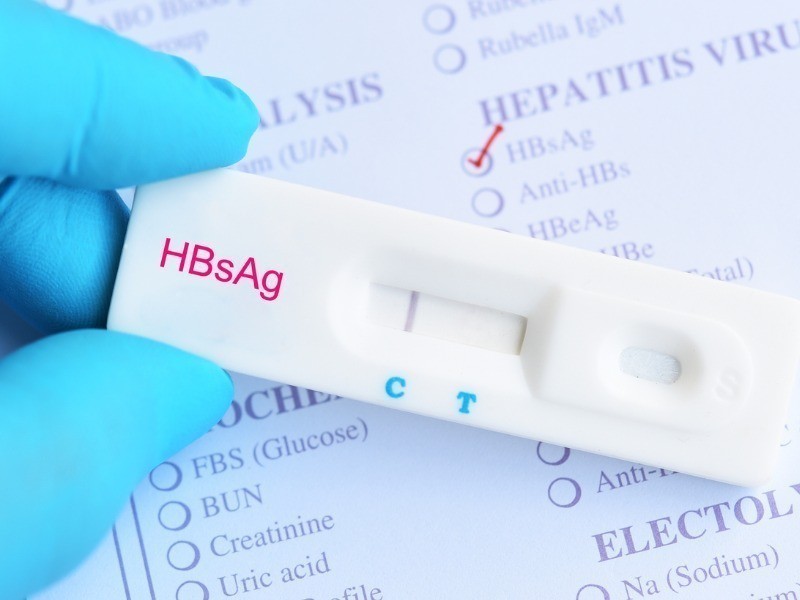
POCT HBsAg
POCT – HBsAg¹
Related tests
¹Rapid Diagnostic Test – HBsAg
¹Rapid Test of HBsAg
Proficiency Testing
- Lyophilized human serum of 200uL.
- Qualitative.
Available programs:
- POCT – HBsAg (2 Items x 3 Rounds) – EPPHBA23 | EP556
Internal Control
- Lyophilized human serum of 200uL.
- Up to 2 years of validity at < 0⁰C.
- Qualitative.
Available programs:
- POCT – HBsAg 2x1xmonthly (2 Levels x 1 Item) – CIPHBA21M | CI129
- POCT – HBsAg 2x1xn (2 Levels x 1 Item) – CIPHBA21N | CI129
Related Programs
-
CL
Anti-HBC – Immunology
Anti-HBc IgG Anti-HBc IgM Total Anti-HBc Related test Hepatitis B Antibodies against the hepatitis B virus core.
EPCLÍNICO -
CL
Anti-HAV – Immunology
Anti-HAV IgG Anti-HAV IgM Total Anti-HAV Related Test Hepatitis A
EPCICLÍNICO -
CL
HBV – Molecular Biology
The program includes PCR-based molecular testing. The samples contain the complete genome of the microorganism. HBV-DNA
EPCICLÍNICO