Those interested in participating in the pilot round can sign up for participation until July 23rd
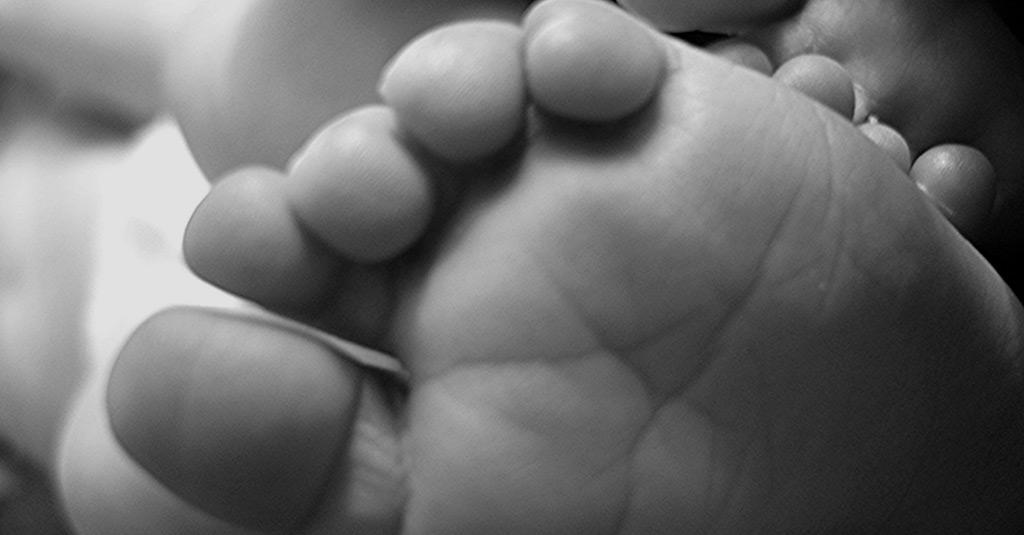
“Neonatal heel prick” is the popular name given to the Guthrie Test, carried out with the aim of early diagnosis in newborns of some diseases that can cause serious complications over the years.
On May 26, 2021, in Brazil, Law nº 14.154/2021 was enacted, which increases the number of diseases detectable by the heel prick test performed on newborns to improve the National Neonatal Screening Program (PNTN).
Issuing reliable reports for screening analyzes is essential with a focus on prevention, early intervention and permanent monitoring of individuals detected with the diseases. Performing Proficiency Testing (PT) – also known as External Quality Control – regularly and in conjunction with the application of Internal Control, promotes reliability in analytical routines.
Controllab has External and Internal Control for Neonatal Screening and, in August, this scope will be expanded with the inclusion of new tests:
• MCAD deficiency
• Lysosomal Diseases
• Acylcarnitines Profile
Currently, there are controls for the following area exams:
• 17OH Progesterone
• Amino acids
• Anti-Cytomegalovirus IgM
• Anti-HIV
• Anti-Rubella IgM
• Anti-Toxoplasma IgM
• Anti-Trypanosoma cruzi
• Biotinidase Activity
• G6PD Activity
• Assessment of Hemoglobins
• Phenylalanine (PKU)
• Total Galactose
• T4
• Immunoreactive trypsin
• TSH
Those interested in participating in the pilot round, which will be sent free of charge on August 9th, can choose to participate in this link until July 23rd.
All tests offered by Controllab follow the criteria of ABNT NBR ISO/IEC 17043. By participating in the PT, the laboratory will be able to evidence the accuracy of the analyzes and the reliability of the results issued in its routine.
To learn more, access the Controllab website, contact us by e-mail customer service@controllab.com or by phone +55 (21) 3891 9900.