Background - Aim
Laboratories employ performance monitoring systems, with quality indicators (QI) playing a key role. Pre-analytical errors, responsible for up to 70% of laboratory errors, require continuous monitoring through QIs. Brazil’s Laboratory Indicators Benchmarking Program, launched in 2006, includes 400 laboratories from 17 countries and 180 indicators, 42 of which focus on pre-analytical QIs. This study aimed to evaluate the performance of laboratories in this program, focusing on seven pre-analytical QIs harmonized by the IFCC WG-LEPS (Working Group on Laboratory Errors and Patient Safety).
Methods
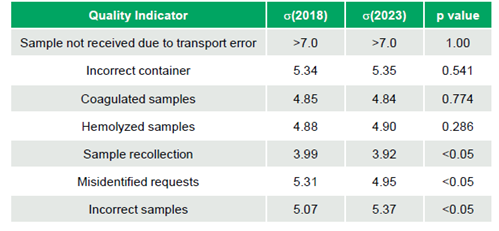
The study evaluated seven pre-analytical indicators: Sample Recollection, Sample Not Received due to Transport Error, Collection Error (Incorrect Sample and Container), Coagulated Samples, Hemolyzed Samples, and Patient Identification Error (misidentified requests). Median values for 2018 and 2023 global data were accessed, and performance was compared using sigma (σ) metrics for the 50th percentile to detect differences.
Results
Analysis showed no significant differences between 2018 and 2023 for four of these seven pre-analytical indicators (Sample Not Received due to Transport Error σ >7.0 to >7.0, Incorrect Container σ 5.34 to 5.35, Coagulated Samples σ 4.85 to 4.84, Hemolyzed Samples σ 4.88 to 4.90; p-values 1.00, 0.541, 0.774, 0.286, respectively). However, statistically significant differences (all p-values <0.05) were observed for three indicators: Sample Recollection (σ 3.99 to 3.92), Misidentified Requests (σ 5.31 to 4.95), both with decreased performance, and Incorrect Samples (σ 5.07 to 5.37), which improved (Table 1).
Conclusions
Although some statistical variations were noted, the sigma performance metrics for most indicators remained consistent between 2018 and 2023, highlighting the stability of data from the benchmarking program. The results suggest that participating laboratories may not be actively implementing or may not be seeing significant improvements in pre-analytical processes. This study emphasizes the importance of sustained improvement efforts, particularly in sample recollection, to enhance laboratory performance.
References
Plebani M, Sciacovelli L, Aita A, Pelloso M, Chiozza ML. Performance criteria and quality indicators for the pre-analytical phase. Clin Chem Lab Med. 2015 May; 53(6): 943-8. doi:10.1515/cclm-2014-1124. Erratumin: Clin Chem Lab Med.2015Sep1;53(10): 1653. doi:10.1515/cclm-2015-7000. PMID: 25719322.
Sciacovelli L, Lippi G, Sumarac Z, Del Pino Castro IG, Ivanov A, De Guire V, Coskun C, Aita A, Padoan A, Plebani M; Working Group “Laboratory Errors and Patient Safety” of International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Pre-analytical quality indicators in laboratory medicine: Performance of laboratories participating in the IFCC working group “Laboratory Errors and Patient Safety” project. Clin Chim Acta. 2019Oct; 497:35-40. doi: 10.1016/j.cca.2019.07.007. Epub2019 Jul 8. PMID: 31295446.
Plebani M, Sciacovelli L, Aita A, Padoan A, Chiozza ML. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Chim Acta. 2014May15;432: 44-8. doi: 10.1016/j.cca.2013.07.033. Epub 2013 Sep 5. PMID: 24012653.
