
MORE SAFETY FOR YOUR LABORATORY
Identify and eliminate errors
inherent in the process
The Internal Control (CI) is responsible for the continuous monitoring of the reproducibility of the laboratory analytical phase, identifying and eliminating errors inherent to the process of analysis of quantitative and qualitative tests. Its purpose is to keep the variability of the test analysis process under control and to offer an opportunity to improve the activities developed in the laboratory.
By enrolling in the internal control programs, the participating laboratory will have access to the service via CI ONLINE, a powerful tool for the management of internal quality control, analysis and treatment of deviations related to batch variation, stability of reagents and calibrators, as well as such as the inaccuracy of the analysis process and its performance over time.
Controllab Catalog
Get to know the broad portfolio of tests covered by quality control and sign up to monitor the quality of tests performed in your routine.
Controllab’s Internal Control materials are considered third-party controls. Including interlaboratory valuation in different systems. These multiple valuations allow the laboratory to detect more sensitive variations, which are not perceived in the controls of the manufacturers of the analytical system.
Benefits of Controllab’s Internal Control Program
It unifies and monitors all daily activities of internal quality control
Enables the use of Internal Control materials from other suppliers available on the market and developed by the laboratories themselves
Fully automate your routine internal controls with your Laboratory Information System (LIS)
The laboratory can compare its performance, in each routine, with that of other similar laboratories
Most samples are previously valued through the Proficiency Testing or interlaboratory, which gives them an important reference for their valuation process.
Samples produced with the most modern quality standards in Good Manufacturing Practices (GMP), tested and approved in accordance with the requirements of ABNT NBR ISO/IEC 17043 and ISO 13528 for homogeneity and stability carried out in accredited Controllab testing quality control laboratories according to ISO/IEC 17025 (CRL0586)
In order to ensure metrological traceability, allowing instrument calibration and method validation, Controllab uses Certified Reference Materials (CRM) produced by itself with recognition and competence established in ABNT NRB ISO 17034:2017 (PMR 0009)
ACCREDITATIONS AND
RECOGNITIONS
The samples that integrate the Controllab Internal Control Program go through a rigorous production process, homogeneity and stability. This commitment has provided some recognition:
According to the scope
published at www.inmetro.gov.br
Analytical Process Control
At CI ONLINE the laboratory can use Controllab samples (valued by interlaboratory), the internal control materials from other suppliers available on the market and those developed by the laboratories themselves with visualization of the behavior of the results in a single Control Center. This flexibility generates more practicality and productivity in the routine analysis and monitoring processes.
Facilitates failure prevention and resolution
Issues approvals, alerts and rejections of analytical runs in real time for laboratory tests.
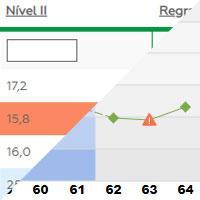
Performance monitoring
Uses Levey Jennings graphics for tests and equipment.
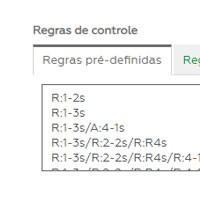
Control rules
It allows the choice of control rules (alerts and rejection) that are most appropriate to the routine: multiple Westgard rules, Percentage, DP or Fixed Limit.
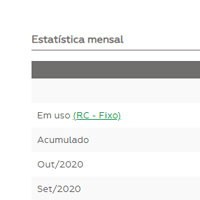
Summary and statistical information
Displays summary and statistical information for monitoring and decision making.
Tracks actions completely
It allows the inclusion of actions and comments with access to the documentation: package insert of reagents/kits and control materials; and equipment manuals.

Meets laboratory accreditation standards
ISO 17025, ISO 15189, PALC, ONA, DICQ and others.

Prove your credibility and quality
The evidence is quick and practical for regulatory bodies, certifiers and accreditors such as INMETRO, ANVISA, VISA e MAPA.
Errors in laboratory analysis, in most cases, occur due to systemic failures. With CI ONLINE, Controllab provides more confidence in your results and laboratory reports.
COMPLETE MANAGEMENT OF INTERNAL QUALITY CONTROL AND INTERLABORATORIAL COMPARISON
CI ONLINE + Integration
CI ONLINE works via web on any device, such as computers, tablets and smartphones, allowing the user the mobility to use anywhere with security, confidentiality and scalability.
Another advantage is that it allows the Integration of any Laboratory System, sending the results and receiving the information of approval or not of the analytical run. The use of “CI ONLINE Integration” fully automates the internal quality control from the receipt of samples to the approval of analytical runs, increasing the safety and productivity of your laboratory.

How CI ONLINE + Integration will make your routine easier
1 Sample
By reading the sample through the Internal Control barcode, your laboratory’s LIS automatically identifies the control.
2 Laboratory LIS
The integration of LIS, Middleware and equipment, speeds up the sending of control data since it does not require typing.
3 Middleware
Through this exchange, all approvals, alerts and rejections are identified and transmitted in real time.
4 CI ONLINE
Demonstrates data in real time for any device, allowing agility in the actions of the analytical routine.
CI ONLINE: MONITORING OF THE ANALYTICAL SYSTEM AND ONLINE CONTROL CENTER
Analyze the behavior of your routine data anywhere
The control center allows the user to analyze anywhere (inside or outside the laboratory) the behavior of the data in their routines. According to acceptance rules pre-configured by the laboratory, the system issues alerts that signal data outside its specifications.
Actions such as a new valuation period, data deletion/alteration, control rules changes and comments, can be performed in the Data Entry area at any time by the user. All history is recorded to ensure full traceability of your actions.
In CI ONLINE the laboratory defines for each test which control rules should be used. In this process, the laboratory, with its quality specification, must register the reference values and/or the multiple rules necessary to control its routine.
The system also allows the user to follow their data through the Levey-Jennings graph in an interactive way, including actions and comments during the routine, and to view the application of multiple rules.
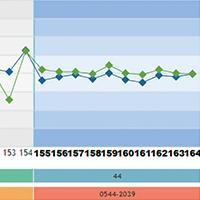
In addition to the graph monitoring, the user has access to the statistical summary with monthly analysis, accumulated (from all data since the beginning of the use of the control material) and “in use” (data statistics for the configured control rule) and statistics per batch of the reagent kit used.
Vitros® system users also have specific statistics per slide generation. Data is easily viewed and any sudden or gradual change in performance can be identified immediately
CI ONLINE has a report that correlates the batch of reagent kits, the period of use and the number of times that it was applied.
It has a resource for identifying recurrent causes, which helps in verifying the effectiveness of the implemented actions. During the analysis of violations of the control rules and/or the Allowed Random Error, if the reported cause has already been used for the same exam/test material, the system will indicate the recurrence(s), equipment/kit/material control/lot(s) and associated reason(s).
Pioneering spirit, trust, and innovation that transform analytical results.
Access our corporate brochure and see how we make a difference.






