The process provides more precision in the critical analyzes carried out by the laboratory.
Among the recommended practices for monitoring internal quality control, the valuation process stands out. This process provides more precision in critical analyses, as it is based on the laboratory’s own metrics (analytical reality), making monitoring more effective.
Valuation strategically contributes to routine analytical decisions. For a better understanding of this process, we will highlight in this article the concepts of this practice, providing the necessary knowledge to adopt it in the routine, resulting in more efficient processes, with higher quality and with more safety for the patient.
Here you can see:
- What is a valuation process?
- How is this process done?
- When to start the valuation process?
- What to do after valuation?
- If my material has a short shelf life, how can I evaluate it?
- How can CI Online help your laboratory in the valuation process?
You will find the answers in this explanatory video and also in the continuation of the following text.
What is a valuation process?
It is a process of generating internal control result ranges by the laboratory, based on the metrics themselves. These ranges represent actual process variation.
How is this process carried out?
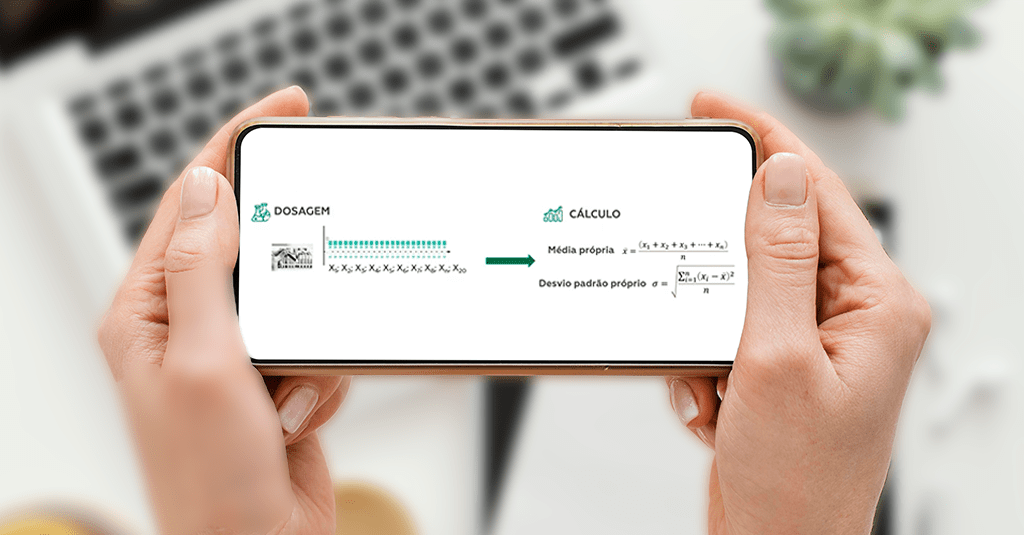
The process is divided into two stages. The first is the dosing step and the second is the calculation step.
The dosing step consists of carrying out between 10 and 20 successive measurements of the internal control material. This allows obtaining data that will be used in the calculation of means and standard deviations, in the next step.
In the calculation stage, we used the values obtained in the dosages to measure the mean and standard deviation. It is important to assess whether the dispersion obtained in the standard deviation is compatible with the laboratory’s quality specification.
More details on planning the quality specification can be obtained in the book Management of the Laboratory’s Analytical Phase – How to ensure quality in practice (vol. II), available free of charge from Controllab in the Learning menu of the website.
When to start the valuation process?
To avoid discontinuing the process, ideally, the valuation of the new internal control batch kit should be carried out while the current batch does not expire. So, it is worth noting that it is essential to evaluate the dispersion behavior of the new batch with the values of previous batches through a historical analysis.
What to do after the valuation process?
After completion of the assessment, the laboratory can apply control rules (Westgard Rules), which act as criteria for judging whether a run is within or outside the acceptance range. Producing your own values provides a narrower acceptability range, providing tighter control.
If my material has a short shelf life, how do I carry out the valuation?
Liquid internal control materials, such as, for example, in hematology and flow cytometry, have a short validity compared to lyophilized materials. Therefore, the valuation process can be adopted in a way that optimizes its available time of use.
One possibility is to carry out the valuation in four days, with five daily measurements, to obtain more data in a shorter time, taking care to analyze at different times of the day to avoid the propagation of specific errors.
How can CI ONLINE help your laboratory in the valuation process?
CI Online is an internal quality control management tool offered by Controllab. It allows you to automatically monitor and value internal control.
In CI ONLINE, the laboratory can use Controllab samples (valued by an interlaboratory), in addition to internal control materials from other suppliers available on the market and those developed by the laboratories themselves, with the visualization of the behavior of the results in a single Control Center .
This flexibility generates more practicality and productivity in the processes of analysis and monitoring of the laboratory routine. Another advantage of this tool is that it allows integration with any Laboratory System, enabling the sending of results and the receipt of approval or disapproval information of the analytical run.
Get in touch with Controllab and learn more about how CI Online can help your routine!


