Background - Aim
Hepatitis C virus (HCV) infection remains a global health challenge, causing chronic liver disease and approximately 400,000 deaths annually, with a high burden in low- and middle-income countries (LMICs). Anti-HCV detection is a standard screening tool, and point-of-care tests (POCT) offer rapid results to support timely decisions. External Quality Assessment Programs (EQAP) are vital for ensuring the reliability and comparability of test results.
Methods
Data from 2010 to 2024, spanning four annual rounds, were analyzed. Participants evaluated four lyophilized and/or liquid quality control samples per round. Metrics included participation, adequacy rates (%A), inadequacy rates (%I), specificity (SP), and sensitivity (SE).
Results
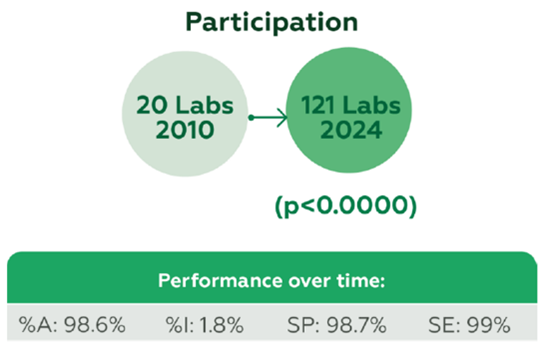
A total of 290 participants contributed 16,828 datasets, with participation growing from 20 laboratories in 2010 to 121 in 2024 (p<0.0000). Performance remained high over time, with overall %A 98.6%, %I 1.8%, SP 98.7%, and SE 99% (Figure 1).
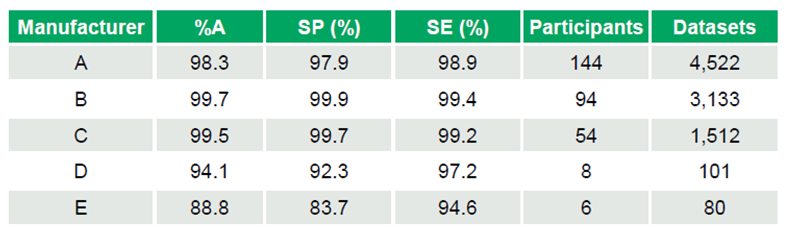
Of 24 manufacturer kits, 18 achieved %A > 98%, while 2 scored below 95%. Performance highlights for commonly used kits include:
- Manufacturer A: %A 98.3%, SP 97.9%, SE 98.9% (144 participants – 4,522 datasets)
- Manufacturer B: %A 99.7%, SP 99.9%, SE 99.4% (94 participants – 3,133 datasets)
- Manufacturer C: %A 99.5%, SP 99.7%, SE 99.2% (54 participants – 1,512 datasets)
Smaller datasets showed lower performance:
- Manufacturer D: %A 94.1%, SP 92.3%, SE 97.2% (8 participants – 101 datasets)
- Manufacturer E: %A 88.8%, SP 83.7%, SE 94.6% (6 participants – 80 datasets) (Table 1)
Conclusions
The growing adoption of POCT for anti-HCV in Brazil is evidenced by increased EQAP participation. High %A, SP, and SE, alongside low %I, confirm the reliability of POCT, matching traditional laboratory test standards. EQAP monitoring ensures robust performance and supports accurate HCV diagnostics.
References
Updated recommendations on simplified service delivery and disagnostics for hepatitis C infection: policy brief. ISBN978-92-4-005269-7(electronicversion). ISBN978-92-4-005270-3 (printversion) ©WorldHealthOrganization 2022.
C.Vassalle, A.Mercuri, S.Masini, S.Antongiovanni, A.Pilo, F.Cantini, G.C.Zucchelli. External quality assurance program in anti-HCV serological detection: an Italian experience. Immuno-analyse & Biologie Spécialisée, Volume19, Issue4, 2004, Pages 235-238, ISSN 0923-2532, https://doi.org/10.1016/j.immbio.2004.05.001.
Carvalho-Gomes Â, Cubells A, Pallarés C, Hontangas V, CondeI, Di Maira T, Peiró S, Sanfélix-Gimeno G, López-Labrador FX, Berenguer M. Apopulation-based screening for hepatitis C antibodies and active infection using a point-of-care testin a low prevalence area. PLoS One. 2020 Feb11;15(2): e0228351. doi:10.1371/journal.pone.0228351. PMID:32045417; PMCID: PMC7012430.