Background - Aim
High-risk HPV causes cervical cancer, a global health challenge. Molecular DNA testing enables early detection, allowing timely treatment, improving screening, stratifying risks, and optimizing follow-ups. Reliable results require standardized laboratory performance. External Quality Assessment Programs (EQAP) ensure accuracy (AC) and harmonization in HPV diagnostics. This study aimed to evaluate the performance of laboratories in an EQAP for high-risk HPV detection, organized by a provider accredited to ABNT NBR ISO/IEC 17043:2011.
Methods
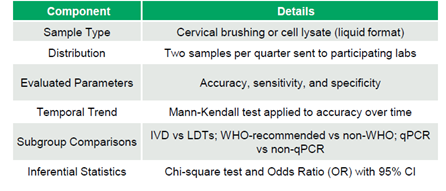
The EQAP consisted of liquid samples from cervical brushing or cell lysate. Participating laboratories received two samples quarterly to evaluate the accuracy (AC), sensitivity (SE), and specificity (SP), which were thoroughly described. Accuracy trends over time were analyzed using the Mann-Kendall test. Sub-group comparisons included in-vitro diagnostic (IVD) kits versus laboratory-developed tests (LDTs), WHO-recommended kits versus others (non-WHO), and qPCR versus non-qPCR methods. Chi-square tests and odds ratios were employed for statistical comparisons (Table 1).
Results
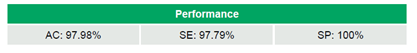
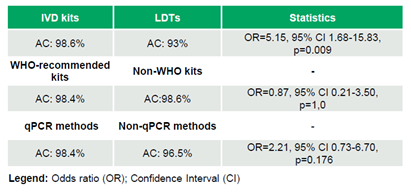
Between 2020 and 2024, 69 labs participated in multiple rounds of the program, with a median of 18 laboratories/round (range: 5–51), generating a total of 692 datasets (Table 2). The laboratories achieved 97.98% (678/692) AC, 97.79% (620/634) SE, and 100% (58/58) SP (Table 3). No significant trends in AC were observed over time (τ=-0.046, p=0.795). IVD kits outperformed LDTs, achieving 98.6% (612/621) AC compared to 93.0% (66/71) (OR=5.15, 95% CI 1.68–15.83, p=0.009). WHO-recommended kits and non-WHO kits demonstrated comparable AC rates of 98.4% (185/188) and 98.6% (427/433), respectively (OR=0.87, 95% CI 0.21–3.50, p=1.0). Similarly, qPCR methods showed similar performance to non-qPCR methods, with AC rates of 98.4% (541/550) versus 96.5% (136/141) (OR=2.21, 95% CI 0.73–6.70, p=0.176) (Table 4).
Conclusions
The High-risk HPV EQAP demonstrated robust diagnostic performance, with IVD kits significantly outperforming LDTs. Both WHO-recommended and non-WHO kits, as well as qPCR and non-qPCR methods, exhibited comparable AC. These findings highlight the quality of HPV diagnostics in participating laboratories and support preferring IVD tests to address the global burden of HPV-related diseases.
References
WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition: Use of mRNA tests for human papillomavirus (HPV)-World Health Organization, 2021.
Human papillomavirus (HPV) nucleic acid amplification tests (NAATs) to screen for cervical pre-cancer lesions and prevent cervical cancer: policy brief-World Health Organization, 2022.
Okada PA, Mitrat S, Rojanawiwat A. External quality assessment program for human papillomaviruses DNA testing in Thailand. Pract Lab Med. 2023 Dec26; 38: e00352. doi: 10.1016/j.plabm. 2023. e00352. PMID:38292923; PMCID: PMC10825476.