Background
Thyroid hormone levels play a crucial role in assessing thyroid function and diagnosing dysfunction. External quality assessment programs (EQAP) aim to evaluate laboratory analytical capabilities, identify differences, and enhance quality.
Aim
This study assesses the performance evolution of laboratories participating in an EQAP for thyroid hormones by a Brazilian proficiency testing (PT) provider accredited by the ABNT NBR ISO/IEC 17043:2011 from 2007 to 2023.
Methods
EQAPs for TSH and free T4 involved four surveys per year with three lyophilized human serum samples per survey. Analyzed data included the number of participants, methods used, mean coefficient of variation (CV), and median CV for different methods.
Results
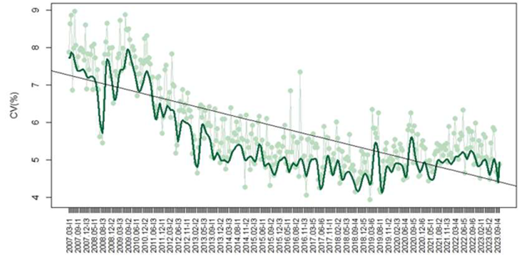
In regard to TSH, 1,101 laboratories contributed 90,288 datasets, with a mean CV decreasing from 7.9% in 2007 to 4.9% in 2023 (p<0.05) (Figure 1). The methods included chemiluminescence (CL) (788/1291 – 61%), electrochemiluminescence (ECL) (389/1291 – 30%), enzyme immunoassay (EIA) (82/1291 – 6%), enzymatic fluorescence (EF) (25/1291 – 2%), and fluorescence (FL) (7/1291 – 1%). The median CV observed for each method were EIA 9.5%, EF 6.5%, CL 5.9%, ECL 4%, and FL 2.7%. Median CVs varied significantly between methods (p<0.05).
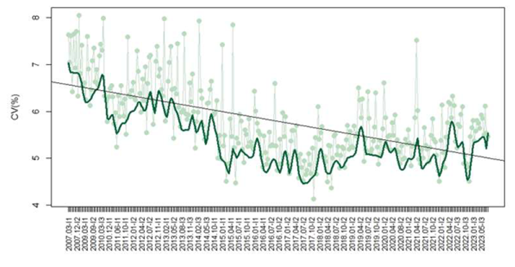
In regard to free T4, 1,345 participants provided 121,922 datasets, with a mean CV decreasing from 7.6% in 2007 to 5.5% in 2023 (p<0.05) (Figure 2). Methods included CL (940/1608 – 58%), ECL (452/1608 – 28%), EIA (157/1608 – 10%), EF (45/1608 – 3%), and FL (14/1608 – 1%). The median CV observed for each method was EF 8.7%, EIA 7.9%, CL 6%, FL 5.1%, and ECL 4.4%. Median CVs varied significantly between methods (p<0.05).
Conclusions
This study revealed variations in CV among testing methodologies. Notably, EIA exhibited the highest CV for TSH, while EIA and EF showed the highest CV for free T4. The consistent decline in CV over time underscores EQAPs’ role in elevating clinical laboratory standards. These findings provide essential insights for clinicians managing patients, emphasizing the importance of considering both results and the laboratory’s testing method.
Referências
Stockigt JR. Guidelines for diagnosis and monitoring of thyroid disease: nonthyroidal illness. Clin Chem. 1996 Jan;42(1):188-92. PMID: 8565225.
Shahangian S. Proficiency testing in laboratory medicine: uses and limitations. Arch Pathol Lab Med 1998; 122:15–30.
Robert WN. Do proficiency testing participants learn from their mistakes? Arch Pathol Lab Med 2002;126: 147–9.