Background - Aim
Syphilis, caused by Treponema pallidum, is a major sexually transmitted infection (STI) with significant health impacts, especially in resource-limited settings. Point-of-care tests (POCT) for Syphilis are essential for rapid diagnosis and timely treatment, offering advantages such as ease of use and applicability outside traditional laboratories. External Quality Assessment Programs (EQAP) are critical for ensuring the accuracy and reliability of POCT results, particularly for complex infections. This study aimed to evaluate the diagnostic performance of laboratories participating in a Brazilian EQAP for Syphilis POCT (immunochromatography) provided by an ABNT NBR ISO/IEC 17043:2011-accredited provider.
Methods
Data from 2010–2024, comprising four annual EQAP rounds with four lyophilized and/or liquid quality control samples per round, were analyzed. Metrics included participation rates, adequacy (%A), inadequacy (%I), sensitivity (SE), and specificity (SP) for commonly used POCT kits.
Results
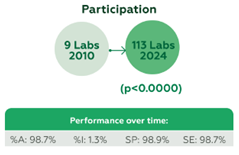
Over 14 years, 348 participants submitted 12,555 datasets. Participation grew from 9 laboratories in 2010 to 113 in 2024 (p<0.0000). Performance metrics remained high: %A 98.7%, %I 1.3%, SP 98.9%, and SE 98.7% (Figure 1). Among 20 tested kits, 16 achieved %A > 98%, with commonly used kits showing robust results:
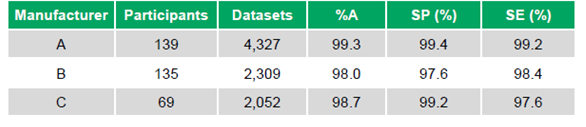
- Manufacturer A: 139 participants, 4,327 datasets, %A 99.3%, SP 99.4%, SE 99.2%
- Manufacturer B: 135 participants, 2,309 datasets, %A 98.0%, SP 97.6%, SE 98.4%
- Manufacturer C: 69 participants, 2,052 datasets, %A 98.7%, SP 99.2%, SE 97.6% (Table 1)
Smaller datasets correlated with lower performance in some kits:
- Manufacturer D: 7 participants, 55 datasets, %A 96.4%, SP 93.3%, SE 100%
- Manufacturer E: 5 participants, 48 datasets, %A 95.8%, SP 96.3%, SE 95.2%
- Manufacturer F: 3 participants, 24 datasets, %A 95.8%, SP 100%, SE 90%
Conclusions
Syphilis POCT adoption in Brazil has expanded significantly, as evidenced by increased EQAP participation and strong performance metrics. EQAPs ensure the reliability of these diagnostic tools, supporting improved STI management and clinical decision-making.
References
Angel-Müller E, Grillo-Ardila CF, Amaya-Guio J, Torres-Montañez NA, Vasquez-Velez LF. Point of care rapid test for diagnosis of syphilis infection in men and non-pregnant women. Cochrane Database of Systematic Reviews 2018, Issue5. Art. No.: CD013036. DOI:10.1002/14651858.CD013036.
Taylor M, Alonso-González M, Gómez B, Korenromp E, Broutet N. World Health Organization global health sector strategy on sexually transmitted infections: an evidence to-action summary for Colombia [Estrategia global de la Organización Mundial de la Salud contra infecciones de transmisión sexual: de la evidencia a la acción. Resumen para Colombia.]. Revista Colombiana de Obstetricia y Ginecología 2017; 68(3):193-201.
Pan American Health Organization. Guidance on Syphilis Testing in Latin America and the Caribbean: Improving Uptake, Interpretation, and Quality of Testing in Di Herent Clinical Settings. Washington, DC. Pan American Health Organization 2015.
Peeling RW, Ye H. Diagnostic tools for preventing and managing maternal and congenital syphilis: an overview. Bulletin of the World Health Organization 2004; 82:439–46.