INDICATORS THAT PROMOTE RESULTS
The Laboratory Indicator Benchmarking Program (PBIL) helps to reduce costs and promote increased efficiency and productivity
How does it help in the day-to-day of the laboratory?
PBIL is a solution for laboratory management that promotes information for more assertive decisions. The tool improves processes, enhances performance, identifies new opportunities and keeps the organization sustainable.
The information generated by the program encourages decision makers to gain a broader view of the results achieved for the process improvement and laboratory sustainability.
Developed in partnership between the SBPC/ML – Brazilian Society of Clinical Pathology/Laboratory Medicine, Controllab partner since 1977.
Concrete evidence of market process performance
By participating in the PBIL, the laboratory identifies whether the efforts and strategies applied in the processes are competitive with their equals, based on hard evidence. This evidence helps to reduce costs, increase efficiency and productivity.
This is because the program uses benchmarked indicators to measure and compare laboratory performance against market performance.

Data analyzed with impair and safety
The indicators contemplated in the PBIL are correlated in all areas, providing a global and systemic view of the process.
A multidisciplinary team (including statistics) reviews data reported in the program of Controllab, that acts as a third party company, imparting impartiality and confidentiality to reported data.
Controllab follows a code of ethical conduct & compliance integrated with national and international laws for general data protection. The program has a detailed manual and description for the reliability and standardization of information.
Benchmarking powered indicators
There are more than 150 indicators available for the laboratory.
To simplify data collection and improve access to information, partner companies integrate the LIS to the PBIL, promoting more agility in processes and reliability in the information available in real time for decision making.

History and tracking for certifications and accreditations
Traceability in the PBIL system favors transparency for accreditations such as ISO 15189, CAP, PALC, DICQ and others, which require the demonstration of objective evidence that supports and assists the planning, monitoring and evaluation of process performance.
Assess whether your laboratory processes are performed competitively
In the Laboratory Indicator Benchmarking Program, the laboratory has a chance to compare with its equals and see if the actions taken over time are significant in relation to the market.
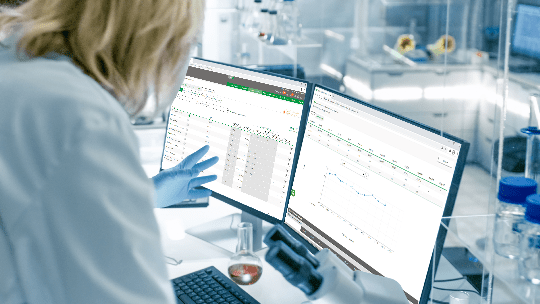
Benchmarking program that collaborates with the improvement of the sector
By participating, the laboratory improves the monitoring of indicators and there is greater effectiveness in management, increasing the level of safety for managers, health professionals and patients.
SBPC/ML and Controllab, in partnership with the Laboratory Indicator Benchmarking Program, are collaborating entities in the QUALISS – Qualification Program of Health Service Providers of ANS – National Agency of Supplementary Health.
Broad and comprehensive
indicator scope
Internationally harmonized indicators aligned with IFCC
Goal Setting to Achieve
Determined Goals
Advisory Group of Experts involved in monitoring the program
Comparison between networks
(support or brands)
Integration with LIS providers to simplify data collection
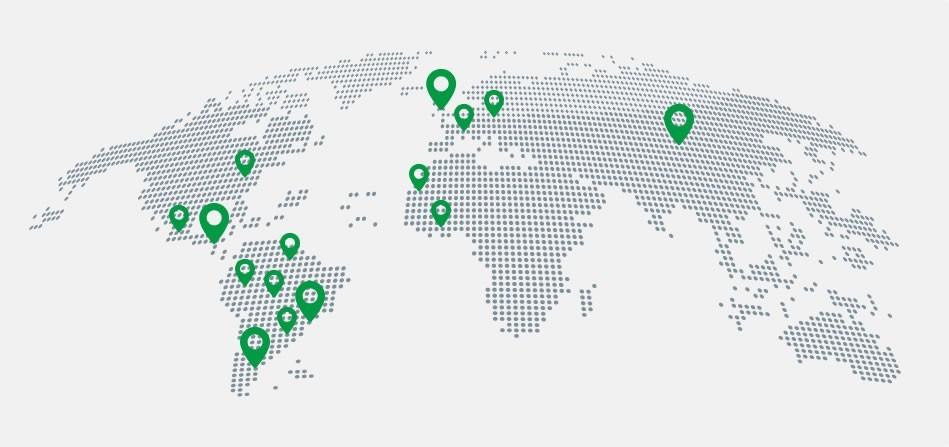
Participant laboratory profiles
~350
Registered
laboratories
16
Countries
Ally in times of crisis management
In times of crisis management, such as the one caused by the Covid-19 pandemic, the Laboratory Indicator Benchmarking Program (PBIL) becomes an ally to managers.
In order to maintain reliable processes, avoid impacts on the quality of the results and protect the reputation of the institutions, the data obtained in the PBIL were fundamental to guide the managers’ decision. Having the measurement and knowledge of the weaknesses and strengths of the laboratory, were determining factors for agile decisions, amid scenarios of uncertainty.

Program operation
Monthly, laboratories receive benchmarking from data reported or obtained directly from the Laboratory Information System (LIS).
Upon registration, the laboratory receives a password to access to the Online System on the Controllab website.
The laboratory fills in the information that will compose its comparison profile (exam amount, target audience, nature, and other information that help it compare it with other organizations with similar characteristics).
Controllab’s statistical team uses laboratory profile information to recommend the best comparison group for their characteristics. The laboratories are compared among their equals.
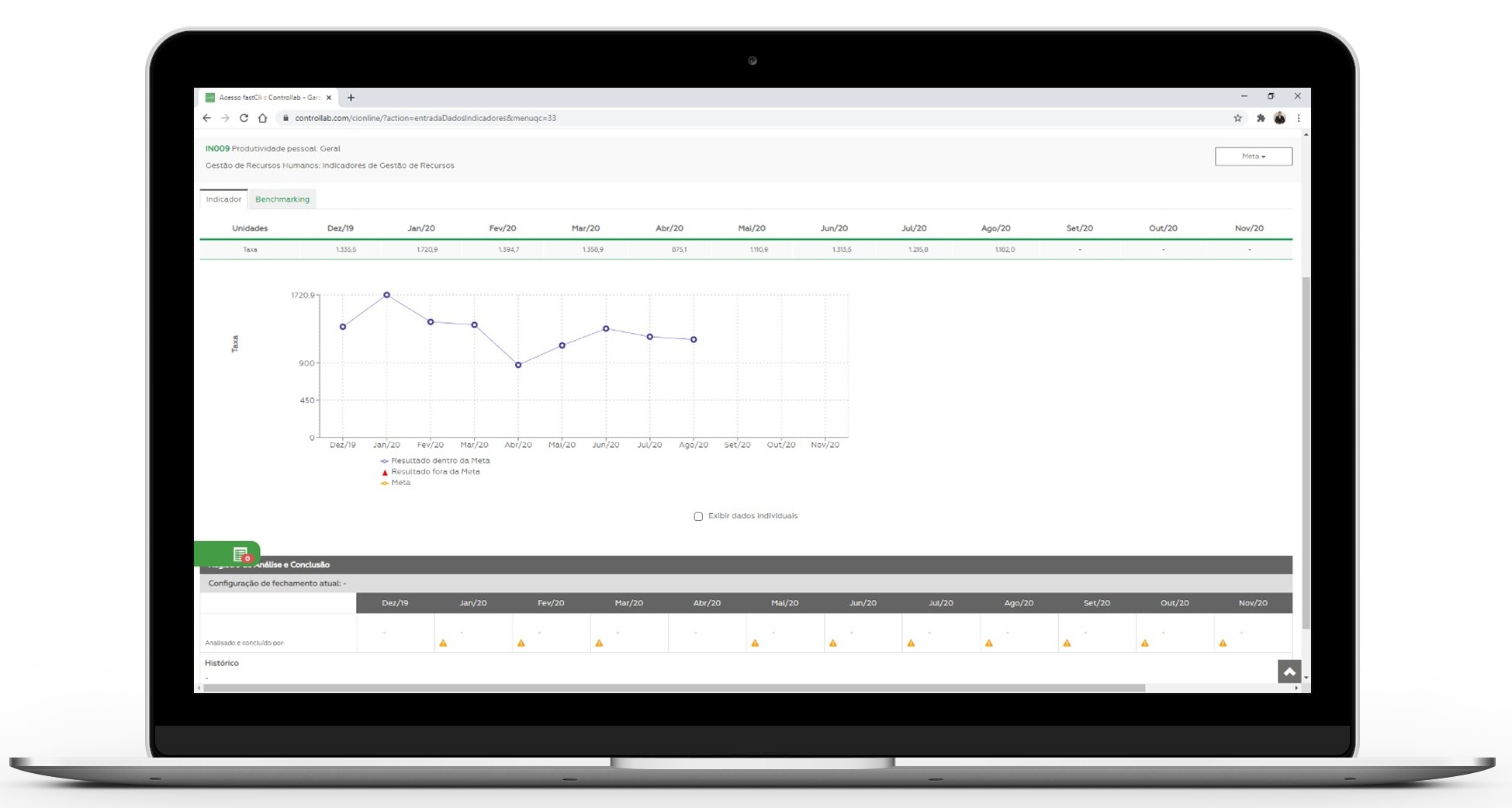
Monthly the laboratory visualizes in real time the market comparison of its results to define the priority actions to the organization’s strategies.
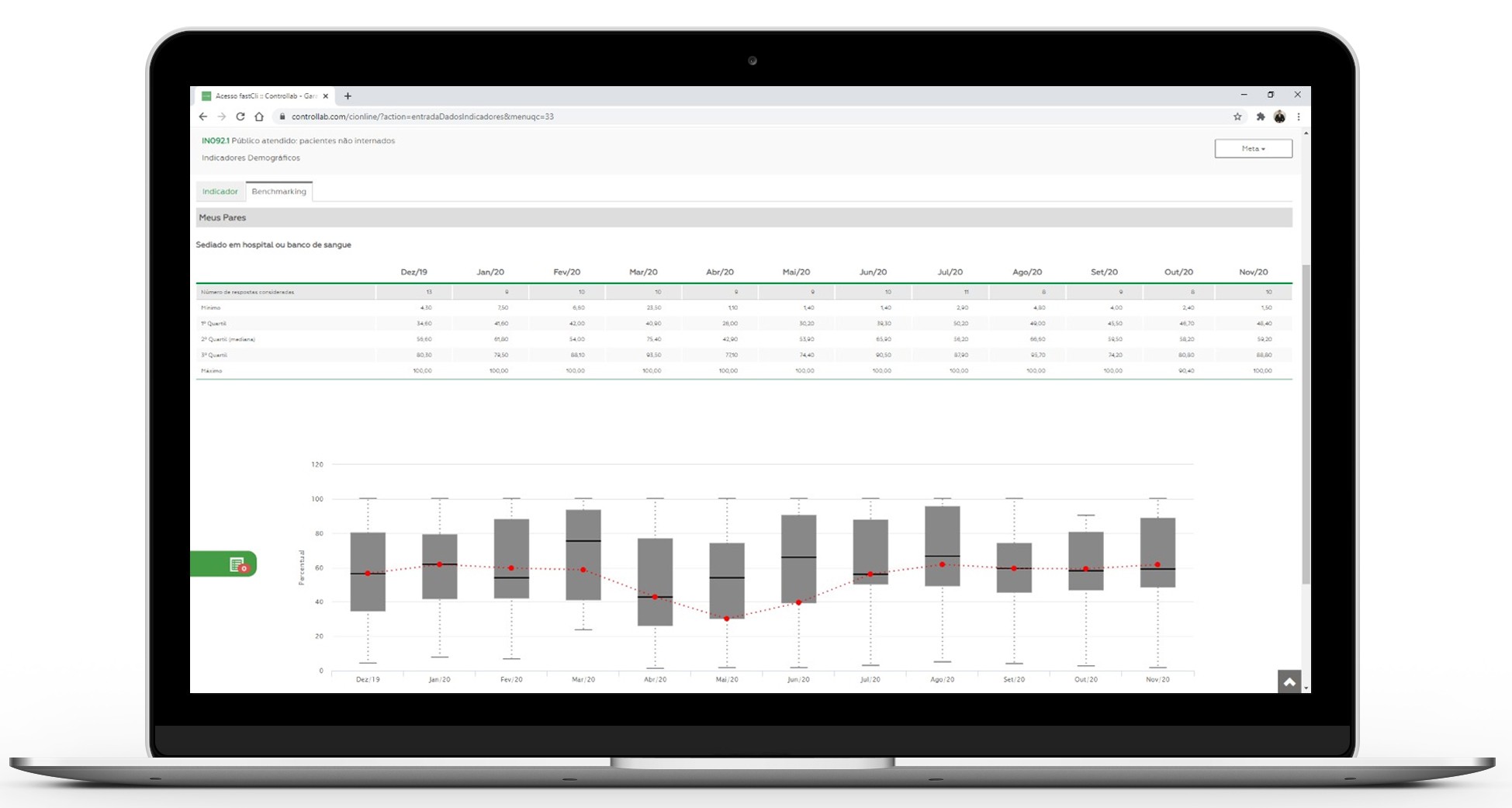
The positioning of the laboratory in the market is indicated in the box plot graph. The Box plot is adopted for the graphical representation of the data because it allows an excellent visualization of the dispersion, symmetry, outlier barriers and outliers, regardless of the way the data is distributed. The Box plot is based on the median and quartiles, which reduces the impact of outliers and allows an excellent exploratory analysis. See how to interpret the result in the Box plot graph.
Relative Equal Ranking and Sigma Metrics are applied to the results, where relevant, to encourage continuous improvement of laboratory processes.
Results in Sigma Metrics: Indicates Process Quality
Ranking: 1st are the best results and 5th the most sensitive results
Quality and Sustainability for the Laboratory
Partinership with LIS companies
LIS developers integrate laboratory systems with PBIL to reduce data collection complexity and improve access to information, promoting faster process processes and reliability of real-time decision-making information.
SEE THE BENEFITS OF INTEGRATING LIS WITH PBIL
Allows
it lets you focus on benchmarking results analysis
Promotes
data
reliability
Helps
reduce data
collection failures
Reduces
time in raw
data monitoring
PBIL PARTNERS
Some developer companies, in addition to integrating LIS, promote the indicators in their systems to contribute to laboratory sustainability. These companies are PBIL partners.

Silvia Yano Gerente de Marketing - Matrix.