PHE – Public Health England has given Controllab the recognition of technical competence and capacity compatible with the collection of licensed cultures
Culture collections are valuable resources that aim to preserve, identify, catalog and distribute the strains deposited in them for use in quality control, support for scientific research, epidemiological studies, as well as for the development and production of bioproducts, vaccines and medicines.
In the routine of the analysis laboratories, the reference strains are fundamental for the quality control of the inputs used in the clinical and veterinary diagnostic exams, in the microbiological analysis of food, water, pharmaceutical products and the environment. They are also of great importance for carrying out studies to validate analytical methods and determine measurement uncertainty.
There are currently 802 collections of cultures in 78 countries and regions* registered in the WDCM (World Data Center for Microorganisms), an international database that groups strain into a unique system of identifiers, facilitating the crossing of information about recommended reference strains for use in quality assurance. In it, it is possible to verify that the same strain has a different designation in each culture collection. For example, the strain Escherichia coli NCTC 11954 is also designated ATCC 35218, CIP 102181, DSM 5923, CCUG 30600, CECT 943 depending on the culture collection.
Among the main collections, the NCTC (National Collection of Type Cultures) stands out, which is the oldest collection for bacteria in the world, founded in 1920. Today, this collection is available for Brazil and other countries in Latin America, through the partnership signed between Controllab and PHE – Public Health England.
The partnership was initiated in 2019, through the licensing for Controllab of the collections NCTC (National Collection of Type Cultures), NCPV (National Collection of Pathogenic Viruses) and NCPF (National Collection of Pathogenic Fungi).
Initially, more than 60 strains (including bacteria and fungi) were made available exclusively by the STRAIN CONTROL Program, which sends strains for prompt delivery in the quantity defined by the laboratory, from the selected microorganisms.
In a natural evolution of Controllab’s production process, which applies the same quality to its production – regardless of the material’s recognition, the strains obtained the seal of ABNT NBR ISO 17034 and currently most of them are available as CRM – Certified Reference Material.
Largest quality control provider in Brazil and Latin America, Controllab has unique know-how in solutions for laboratory quality control, having its technical competence certified by the ISO 9001, 17025, 17034 and 17043 recognitions, Anvisa/Reblas, GMP and the exclusive support of important scientific societies.
Being PHE licensed is another recognition of the technical competence of Controllab, since this process validates the representative’s technical and structural capacity to reproduce with the same quality as the licensing brand.
Making lyophilized strains available for immediate supply and with the recognition of ABNT NBR ISO 17034, is in line with the Controllab mission to continuously provide the best solutions for laboratory quality control. This solution allows laboratories to remain sustainable with resource optimization to insert more traceability and reliability in their analytical process.
“Receiving the lyophilized reference strains certified, at a cost compatible with the reality of the country and with the periodicity defined by the laboratory is invaluable for Microbiology in Brazil and Latin America”, comments Jorge Sampaio, professor of Clinical Microbiology at Faculty of Pharmaceutical Sciences, University of São Paulo and physician responsible for the Microbiology Sector at Fleury Medicina Diagnóstica.
In addition to Brazil, countries such as Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Paraguay, Peru, Suriname, Uruguay and Venezuela can benefit from this initiative. To find out more about the MRC Qualitative Reference Cultures and the STRAIN CONTROL Program, which allows the laboratory to have more time to dedicate itself to exam analysis, with a more practical quality control process and with reduced use of infrastructure resources, access the Controllab website.
* According consult the http://www.wfcc.info/ccinfo/statistics on 02/15/21.
Related News
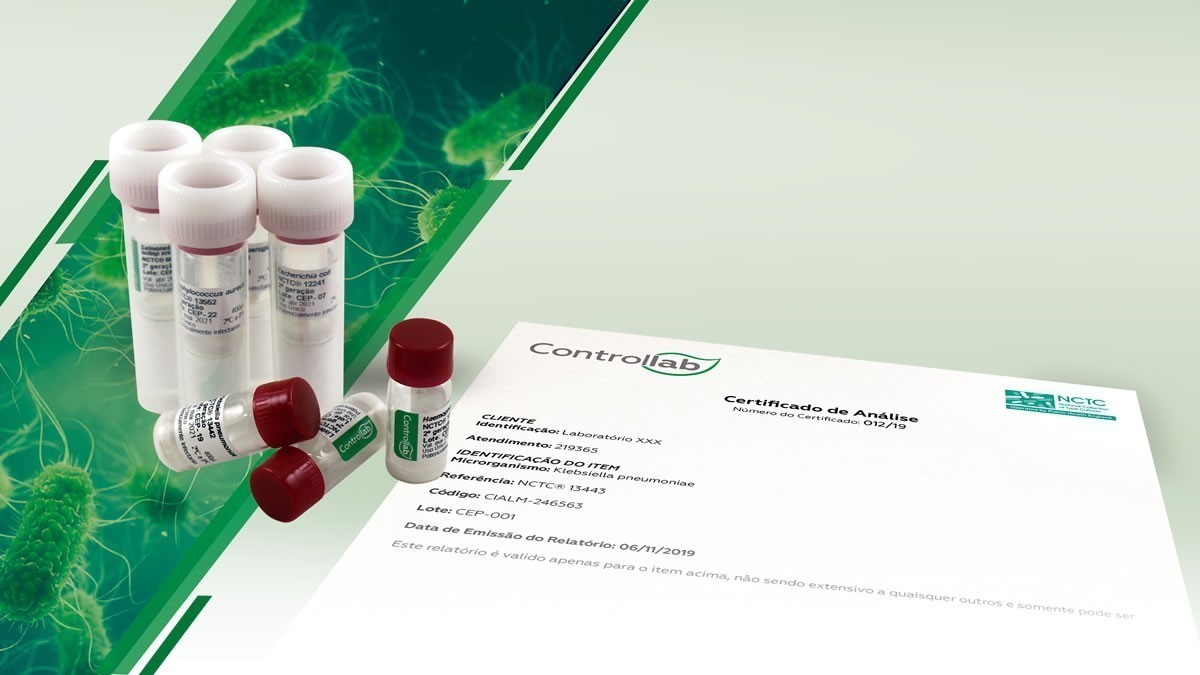
Controllab Strains Control Program Provides Time and Cost Savings
The laboratory receives the vials from the selected microorganisms With the launch of the Strain Control Program, Controllab offers its customers a program that allows the

Controllab expands offer of Certified Reference Materials (CRM)
Accredited by Inmetro’s General Coordination for Accreditation (Cgcre), Controllab produces Certified Reference Material in accordance with internationally accepted criteria and established in the ABNT NBR ISO

Control Strain of Controllab was recognized as Certified Reference Material
Controllab – the largest provider of Laboratory Quality Control in Brazil and Latin America – earned, at the end of August, more recognition for the production